Part A What would the concentration of the resulting glucose solution be if 333 mL of a 30 % (m/v) glucose solution were diluted to a final volume of 1 L with distilled water? Express your answer using one significant figure. να ΑΣφ Cfinal = 0.09 Submit Previous Answers Request Answer * Incorrect; Try Again; 4 attempts remaining
Part A What would the concentration of the resulting glucose solution be if 333 mL of a 30 % (m/v) glucose solution were diluted to a final volume of 1 L with distilled water? Express your answer using one significant figure. να ΑΣφ Cfinal = 0.09 Submit Previous Answers Request Answer * Incorrect; Try Again; 4 attempts remaining
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.43E
Related questions
Question
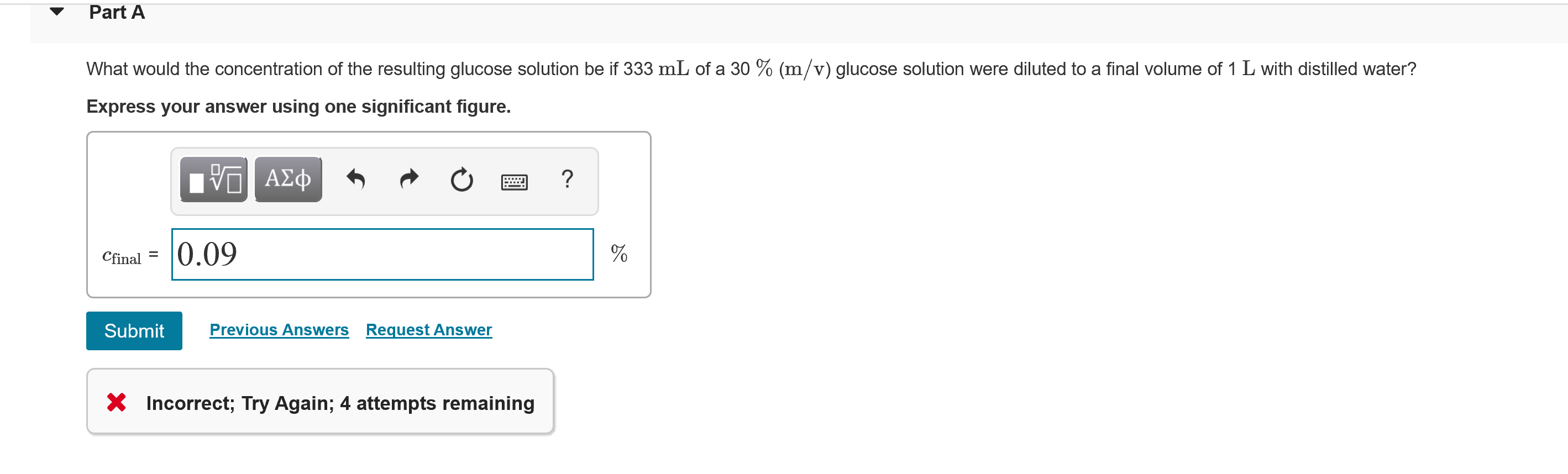
Transcribed Image Text:Part A
What would the concentration of the resulting glucose solution be if 333 mL of a 30 % (m/v) glucose solution were diluted to a final volume of 1 L with distilled water?
Express your answer using one significant figure.
να ΑΣφ
Cfinal = 0.09
Submit
Previous Answers Request Answer
* Incorrect; Try Again; 4 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning