Part A Why can two conversion factors be written for an equality such as 1 m = 100 cm? Two conversion factors cannot be written. Both conversion factors are the same: A conversion factor can be inverted to give a second conversion factor: 1 cm 100 m A conversion factor can be inverted to give a second conversion factor: Submit ovide Feedback 1 m 100 cm A conversion factor can be multiplied by numbers in an equality to give a second conversion factor: 1 m x Request Answer 1 m 100 cm 1 m 100 cm and 100 m 1 cm and 100 cm 1 m 100 cm 1 m and 100 cm x 1 m 100 cm
Part A Why can two conversion factors be written for an equality such as 1 m = 100 cm? Two conversion factors cannot be written. Both conversion factors are the same: A conversion factor can be inverted to give a second conversion factor: 1 cm 100 m A conversion factor can be inverted to give a second conversion factor: Submit ovide Feedback 1 m 100 cm A conversion factor can be multiplied by numbers in an equality to give a second conversion factor: 1 m x Request Answer 1 m 100 cm 1 m 100 cm and 100 m 1 cm and 100 cm 1 m 100 cm 1 m and 100 cm x 1 m 100 cm
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
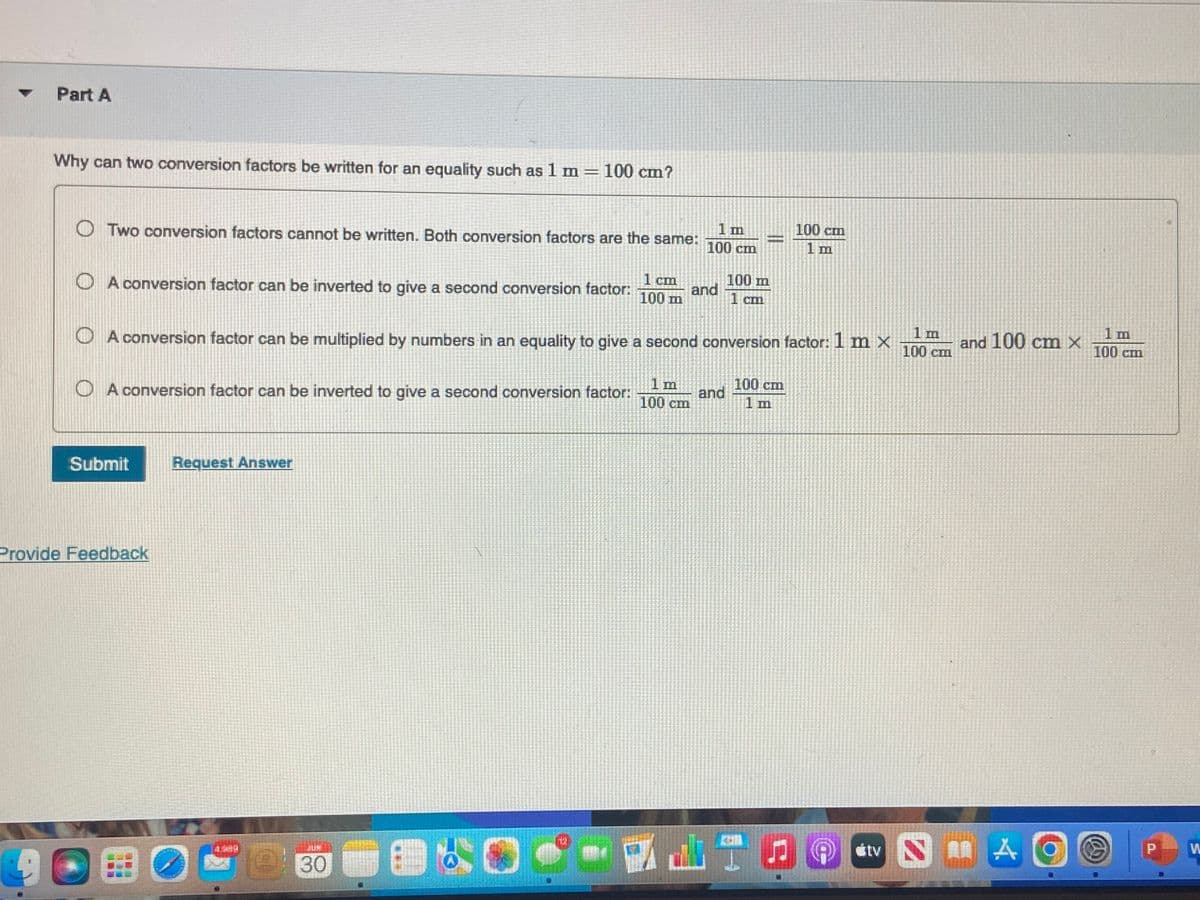
Transcribed Image Text:Part A
Why can two conversion factors be written for an equality such as 1 m = 100 cm?
1 m
100 cm
OTwo conversion factors cannot be written. Both conversion factors are the same:
1 cm
100 m
A conversion factor can be inverted to give a second conversion factor:
A conversion factor can be inverted to give a second conversion factor:
Submit
1 m
100 cm
A conversion factor can be multiplied by numbers in an equality to give a second conversion factor: 1 m x
Provide Feedback
Request Answer
@30
and
100 cm
100 m
1 cm
and
100 cm
1 m
100 cm
1 m
G
#tv
and 100 cm x
寶寶
AO
100 cm
@
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning