Part B Follow these steps to complete the table: 1. Reuse the same test tubes from part A, labeled Fe2* and Fe3*. Be sure they're clean. 2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2*. 3. Add 4 milliliters of iron(II) nitrate to the test tube labeled Fe3+ 4. Add 4 milliliters of potassium iodide to each test tube. 5. Add 1 milliliter of the prepared starch solution to each test tube. 6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction." B IU x x, Font Sizes EE E E E E 囲。 Substances Mixed Iron lon Present Description of the Reaction iron(Il) sulfate, potassium iodide, and starch iron(II) (Fe2+) iron(III) nitrate, potassium iodide, and starch iron(III) (Fe³*.
Part B Follow these steps to complete the table: 1. Reuse the same test tubes from part A, labeled Fe2* and Fe3*. Be sure they're clean. 2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2*. 3. Add 4 milliliters of iron(II) nitrate to the test tube labeled Fe3+ 4. Add 4 milliliters of potassium iodide to each test tube. 5. Add 1 milliliter of the prepared starch solution to each test tube. 6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction." B IU x x, Font Sizes EE E E E E 囲。 Substances Mixed Iron lon Present Description of the Reaction iron(Il) sulfate, potassium iodide, and starch iron(II) (Fe2+) iron(III) nitrate, potassium iodide, and starch iron(III) (Fe³*.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 28QRT
Related questions
Question
100%
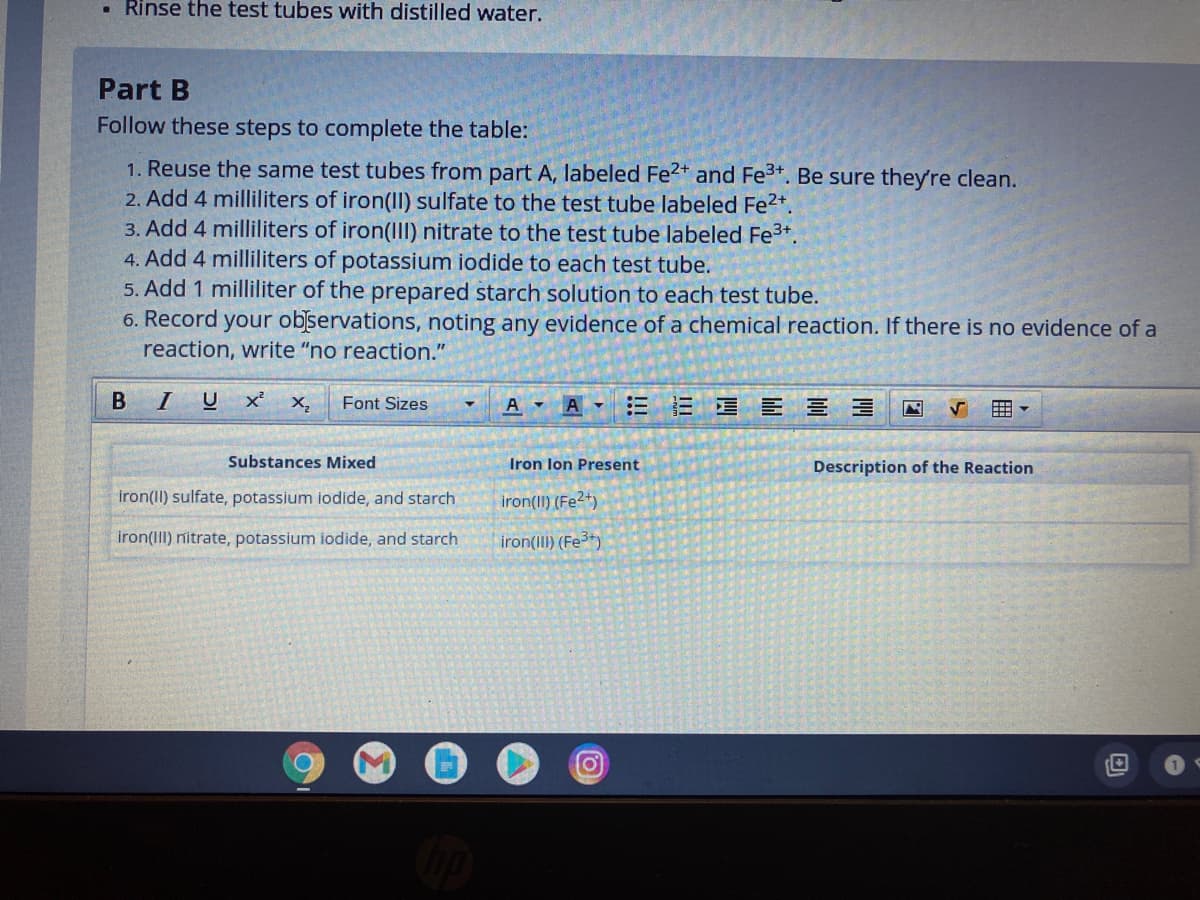
Transcribed Image Text:• Rinse the test tubes with distilled water.
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2*.
3. Add 4 milliliters of iron(II) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium iodide to each test tube.
5. Add 1 milliliter of the prepared starch solution to each test tube.
6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a
reaction, write "no reaction."
В
I U
三E E三 三
X,
Font Sizes
A -
Substances Mixed
Iron lon Present
Description of the Reaction
iron(II) sulfate, potassium iodide, and starch
iron(II) (Fe2+)
iron(III) nitrate, potassium iodide, and starch
iron(III) (Fe)
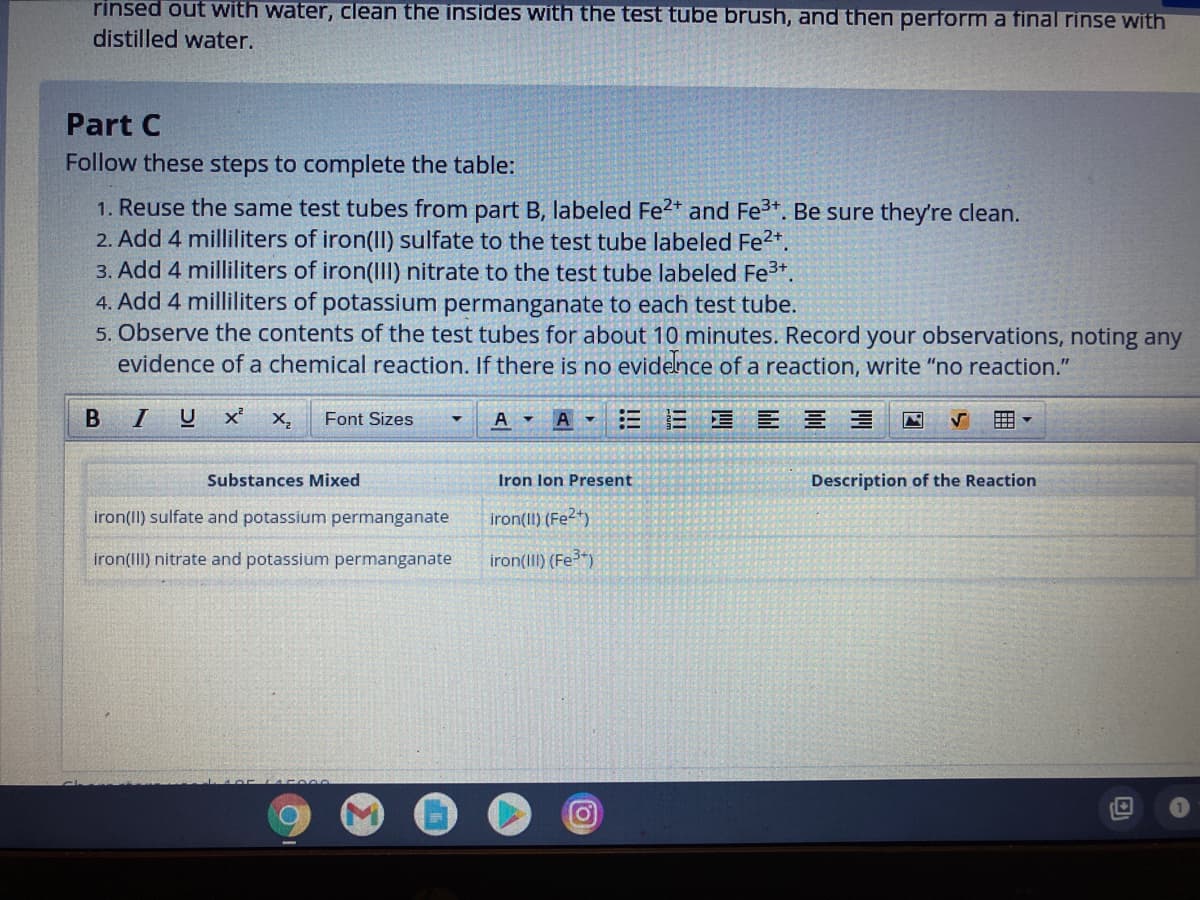
Transcribed Image Text:rinsed out with water, clean the insides with the test tube brush, and then perform a final rinse with
distilled water.
Part C
Follow these steps to complete the table:
1. Reuse the same test tubes from part B, labeled Fe2* and Fe*. Be sure they're clean.
2. Add 4 milliliters of iron(Il) sulfate to the test tube labeled Fe2*.
3. Add 4 milliliters of iron(II) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium permanganate to each test tube.
5. Observe the contents of the test tubes for about 10 minutes. Record your observations, noting any
evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction."
I
X,
Font Sizes
三 E 三 三
出,
Substances Mixed
Iron lon Present
Description of the Reaction
iron(II) sulfate and potassium permanganate
iron(ll) (Fe2+)
iron(III) nitrate and potassium permanganate
iron(III) (Fe*)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning