Part C 2.64x103 kJ to kW h Express the value in kilowatt-hours to three significant figures. ν ΑΣφ kW h Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Part D 4.70x104 J to Cal Express the value in Calories to three significant figures. Cal Submit Request Answer P Pearson nt © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions Con MacBook Air
Part C 2.64x103 kJ to kW h Express the value in kilowatt-hours to three significant figures. ν ΑΣφ kW h Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Part D 4.70x104 J to Cal Express the value in Calories to three significant figures. Cal Submit Request Answer P Pearson nt © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions Con MacBook Air
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 65QAP: Natural gas companies in the United States use the therm as a unit of energy. One therm is 1105 BTU....
Related questions
Question
Convert each conversion between energy units
2.64 x 10^3 kJ to kW•h
express the value in kilowatt hours to 3 sig figs
4.70x10^4 J to Cal
express the value in calories to 3 sig figs
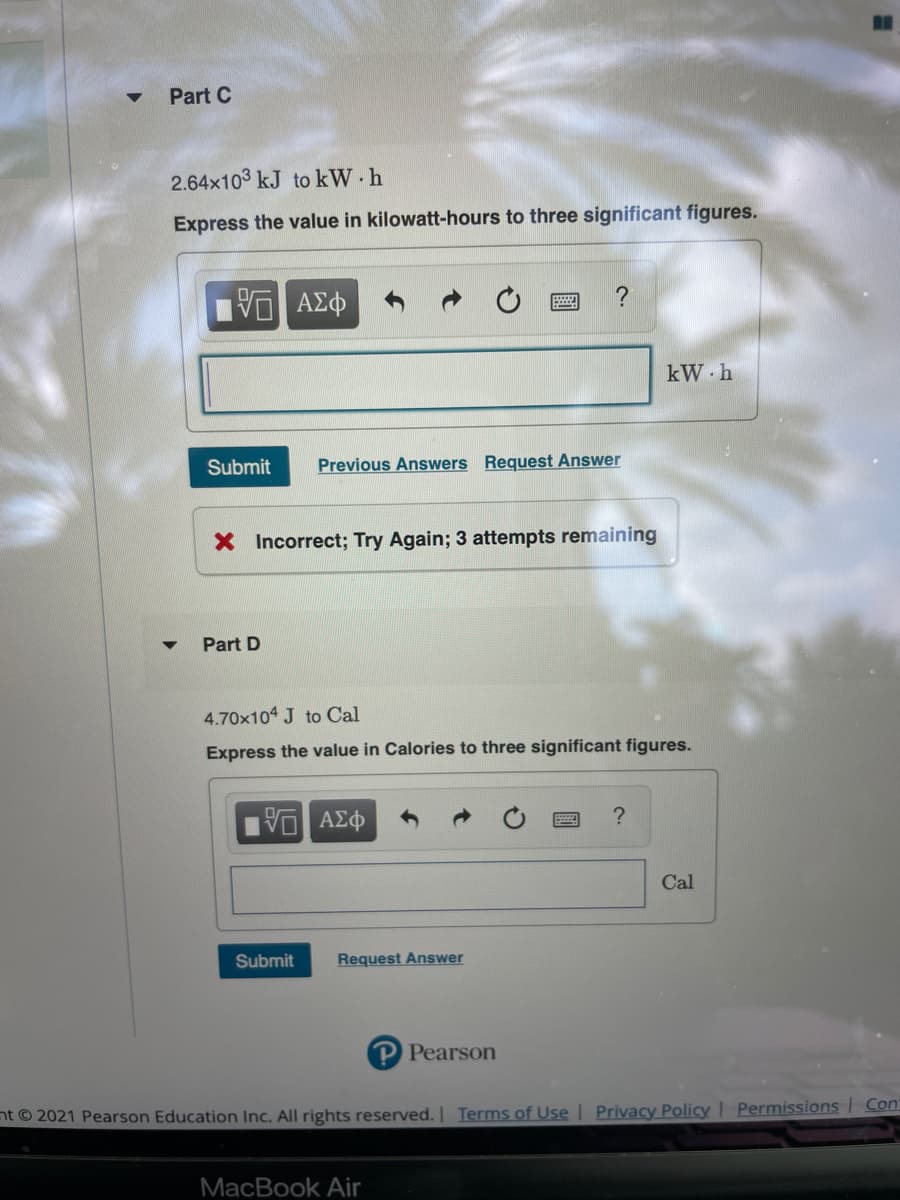
Transcribed Image Text:Part C
2.64x103 kJ to kW h
Express the value in kilowatt-hours to three significant figures.
ΑΣφ
kW h
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 3 attempts remaining
Part D
4.70x104 J to Cal
Express the value in Calories to three significant figures.
Cal
Submit
Request Answer
P Pearson
nt © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions Con
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning