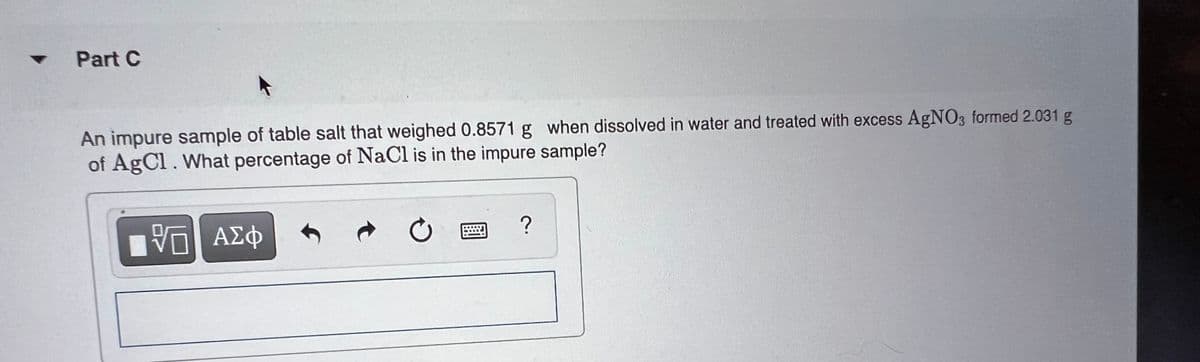
Part C An impure sample of table salt that weighed 0.8571 g when dissolved in water and treated with excess AGNO3 formed 2.031 g of AgCl. What percentage of NaCl is in the impure sample? AZ
Part C An impure sample of table salt that weighed 0.8571 g when dissolved in water and treated with excess AGNO3 formed 2.031 g of AgCl. What percentage of NaCl is in the impure sample? AZ
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 65QAP: Twenty-five milliliters of a solution (d=1.107g/mL)containing 15.25% by mass of sulfuric acid is...
Related questions
Question
Transcribed Image Text:Part C
An impure sample of table salt that weighed 0.8571 g when dissolved in water and treated with excess AgNO3 formed 2.031 g
of AgCl. What percentage of NaCl is in the impure sample?
ΑΣφ
?
Transcribed Image Text:1 of 1
IReview I Constants I Periodic Table
マ
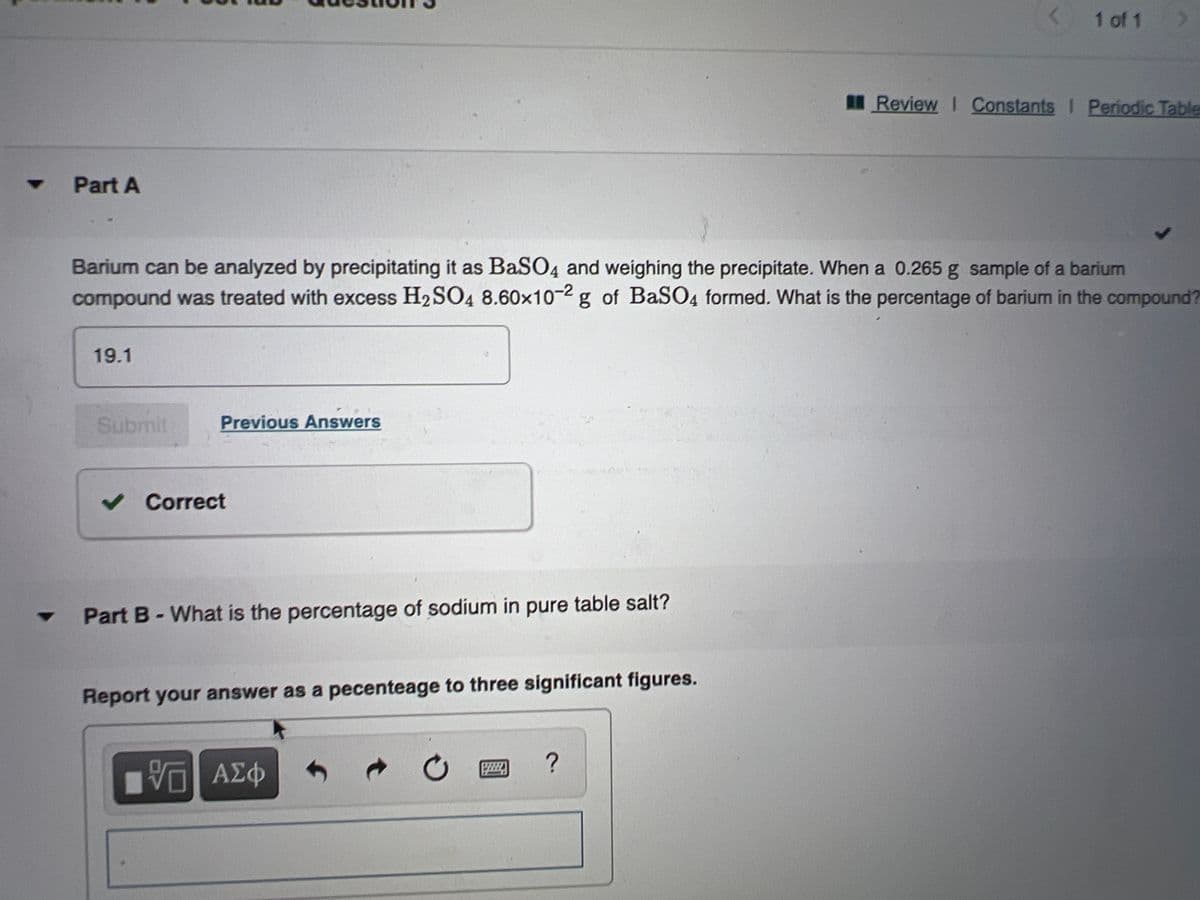
Part A
Barium can be analyzed by precipitating it as BaSO4 and weighing the precipitate. When a 0.265 g sample of a barium
compound was treated with excess H2SO4 8.60x10-2 g of BaSO4 formed. What is the percentage of barium in the compound?
19.1
Submit
Previous Answers
v Correct
Part B-What is the percentage of sodium in pure table salt?
%3D
Report your answer as a pecenteage to three significant figures.
响 AZ4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
