Part C With a power supply able to produce 125 A of current, how long must the nails stay in the electrolysis chamber to receive 8.26x10° C of charge? Express your answer to three significant figures and include the appropriate units. HẢ a) ? Value Units Submit Request Answer
Part C With a power supply able to produce 125 A of current, how long must the nails stay in the electrolysis chamber to receive 8.26x10° C of charge? Express your answer to three significant figures and include the appropriate units. HẢ a) ? Value Units Submit Request Answer
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter31: Determination Of An Equivalent Mass By Electrolysis
Section: Chapter Questions
Problem 1ASA
Related questions
Question
100%
plshelp part C
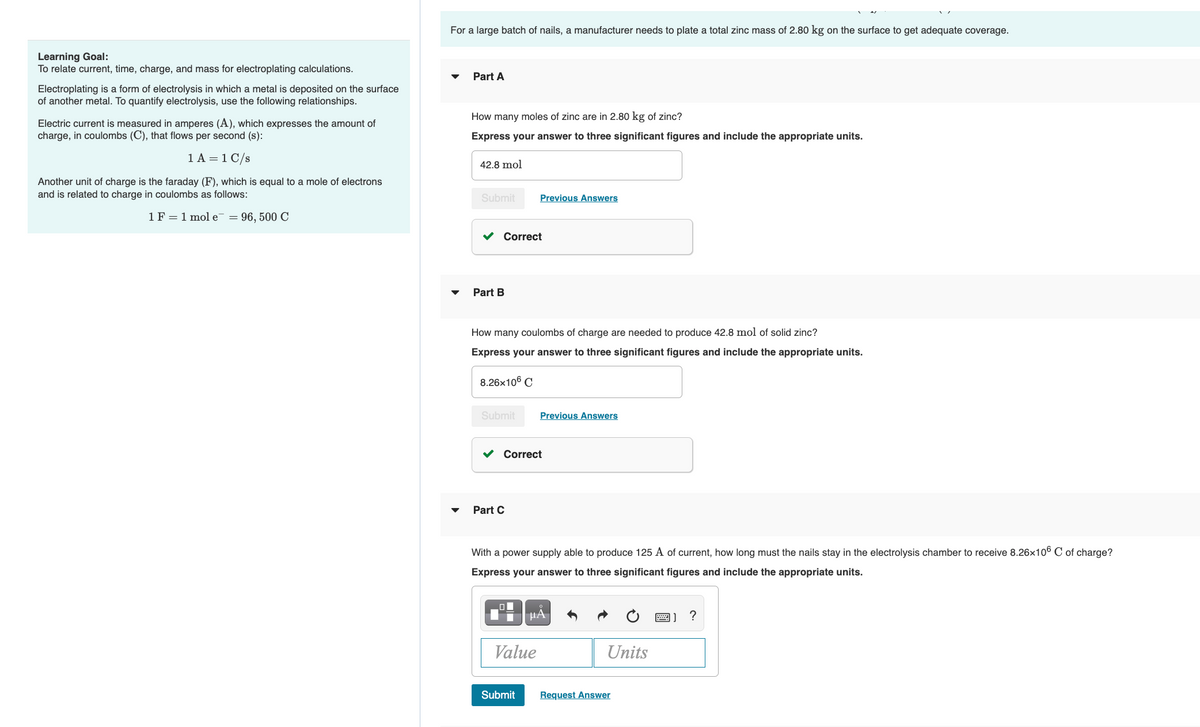
Transcribed Image Text:For a large batch of nails, a manufacturer needs to plate a total zinc mass of 2.80 kg on the surface to get adequate coverage.
Learning Goal:
To relate current, time, charge, and mass for electroplating calculations.
Part A
Electroplating is a form of electrolysis in which a metal is deposited on the surface
of another metal. To quantify electrolysis, use the following relationships.
How many moles of zinc are in 2.80 kg of zinc?
Electric current is measured in amperes (A), which expresses the amount of
charge, in coulombs (C), that flows per second (s):
Express your answer to three significant figures and include the appropriate units.
1 A = 1 C/s
42.8 mol
Another unit of charge is the faraday (F), which is equal to a mole of electrons
and is related to charge in coulombs as follows:
Submit
Previous Answers
1F = 1 mol e¯ = 96, 500 C
v Correct
Part B
How many coulombs of charge are needed to produce 42.8 mol of solid zinc?
Express your answer to three significant figures and include the appropriate units.
8.26x106 C
Submit
Previous Answers
Correct
Part C
With a power supply able to produce 125 A of current, how long must the nails stay in the electrolysis chamber to receive 8.26x106 C of charge?
Express your answer to three significant figures and include the appropriate units.
µA
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
