Part C You carefully weigh out 15.00 g of CaCO3 powder and add it to 60.75 g of HCI solution. You notice bubbles as a reaction takes place. You then weigh the resulting solution and find that it has a mass of 69.60 g. The relevant equation is CACO3 (s) +2HCI(aq)→H2O(1) + CO2(g) + CaCl2 (aq) Assuming no other reactions take place, what mass of CO2 was produced in this reaction? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HA Value Units Submit
Part C You carefully weigh out 15.00 g of CaCO3 powder and add it to 60.75 g of HCI solution. You notice bubbles as a reaction takes place. You then weigh the resulting solution and find that it has a mass of 69.60 g. The relevant equation is CACO3 (s) +2HCI(aq)→H2O(1) + CO2(g) + CaCl2 (aq) Assuming no other reactions take place, what mass of CO2 was produced in this reaction? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HA Value Units Submit
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 38CR
Related questions
Question
Transcribed Image Text:Submit
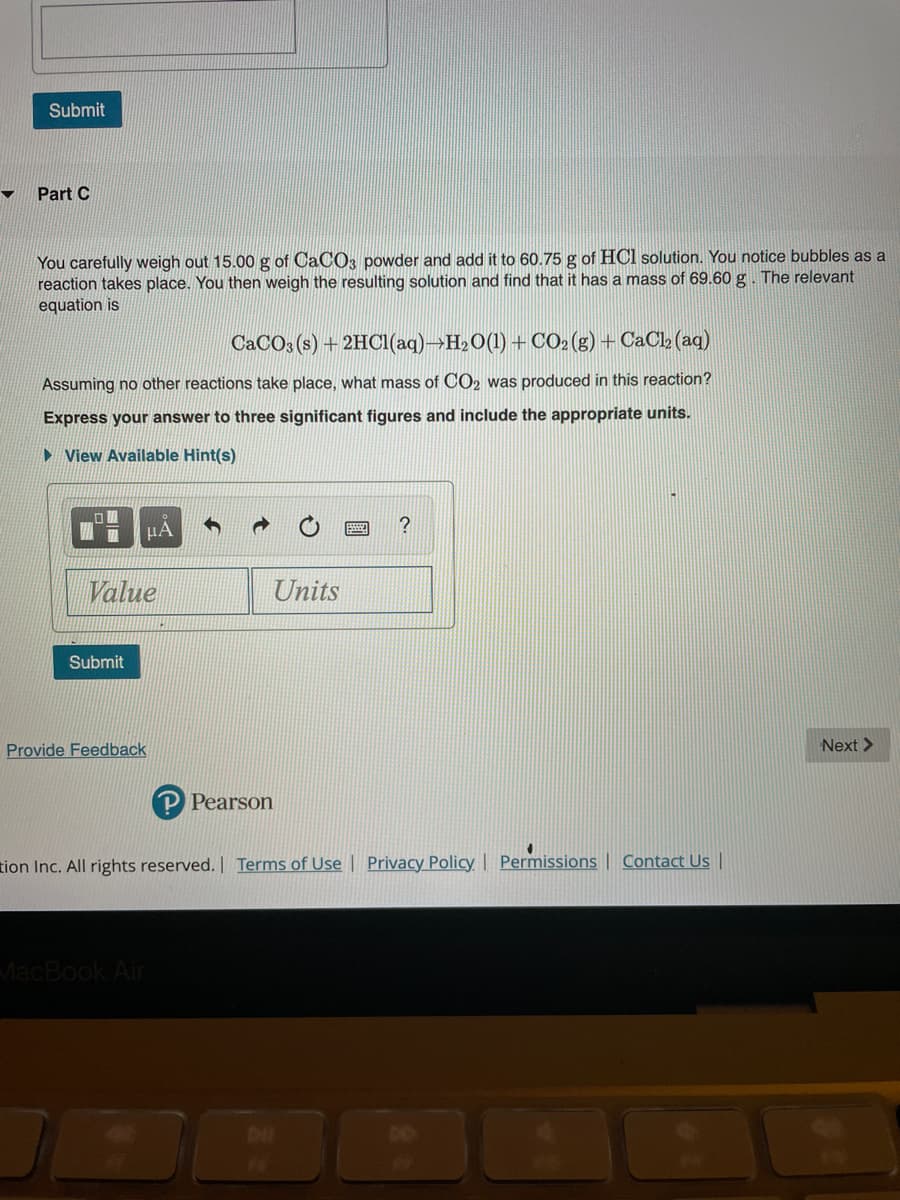
Part C
You carefully weigh out 15.00 g of CaCO3 powder and add it to 60.75 g of HCl solution. You notice bubbles as a
reaction takes place. You then weigh the resulting solution and find that it has a mass of 69.60 g. The relevant
equation is
CACO3 (s) + 2HCI(aq)→H2O(1) + CO2(g) + CaCl» (aq)
Assuming no other reactions take place, what mass of CO2 was produced in this reaction?
Express your answer to three significant figures and include the appropriate units.
► View Available Hint(s)
HA
?
Value
Units
Submit
Provide Feedback
Next >
P Pearson
tion Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
MacBook Air
DIL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning