Consider the chemical reaction aC,H6 + 6CO2 + CH2O → ¿C2H;OH, where a, b, c, and d are unknown positive integers. The reaction must be balanced; that is, the number of atoms of each element must be the same before and after the reaction. For example, because the number of oxygen atoms must remain the same, 26 + c = d. While there are many possible choices for a, b, c, and d that balance the reaction, it is customary to use the smallest possible integers. Balance this reaction. d =
Consider the chemical reaction aC,H6 + 6CO2 + CH2O → ¿C2H;OH, where a, b, c, and d are unknown positive integers. The reaction must be balanced; that is, the number of atoms of each element must be the same before and after the reaction. For example, because the number of oxygen atoms must remain the same, 26 + c = d. While there are many possible choices for a, b, c, and d that balance the reaction, it is customary to use the smallest possible integers. Balance this reaction. d =
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter1: Basic Concepts About Matter
Section: Chapter Questions
Problem 1.87EP
Related questions
Question
6.

Transcribed Image Text:Fertilizer is sold in 100 pound bags labelled with the amount of nitrogen ( N), phosphoric acid ( P205), and potash ( K20) present. The mixture
of these nutrients varies from one type of fertilizer to the next.
For example, a 100 pound bag of Vigoro Ultra Turf fertilizer contains 29 pounds of nitrogen, 3 pounds of phosphoric acid, and 4 pounds of
potash.
Another type of fertilizer, Parker's Premium Starter, has 18 pounds of nitrogen, 25 pounds of phosphoric acid, and 6 pounds of potash per 100
pounds.
Determine the amount of each type required to yield a mixture containing the 101 pounds of nitrogen, 103 pounds of phosphoric acid, and 28
pounds of potash.
The mixture contains
pounds of Vigoro, and
pounds of Parker's.
Hint: Let x1 be the amount of 100 pound bags of Vigoro, and x2 the amount of 100 pound bags of Parker. Set up and solve the system in the
unknowns x1 and x2 and then multiply the solution by 100 to find the number of pounds.
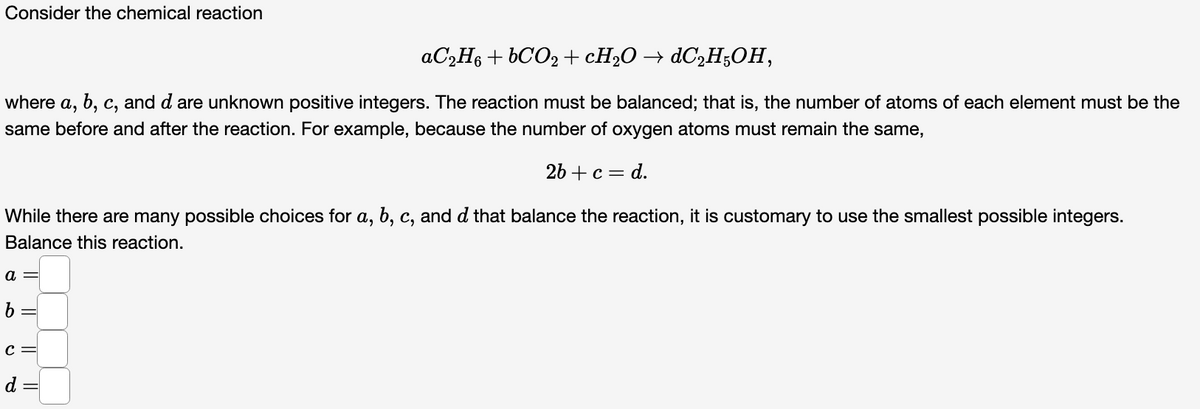
Transcribed Image Text:Consider the chemical reaction
AC2H6 + 6CO2 +CH2O → ¿C2H;OH,
where
a,
b, c, and d are unknown positive integers. The reaction must be balanced; that is, the number of atoms of each element must be the
same before and after the reaction. For example, because the number of oxygen atoms must remain the same,
2b + с — d.
While there are many possible choices for a, b, c, and d that balance the reaction, it is customary to use the smallest possible integers.
Balance this reaction.
a =
b =
d =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning