General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.28QP: Consider the following specific heats of metals. Metal Specific Heat copper 0.385 J/(gC) magnesium...
Related questions
Question
Transcribed Image Text:F2
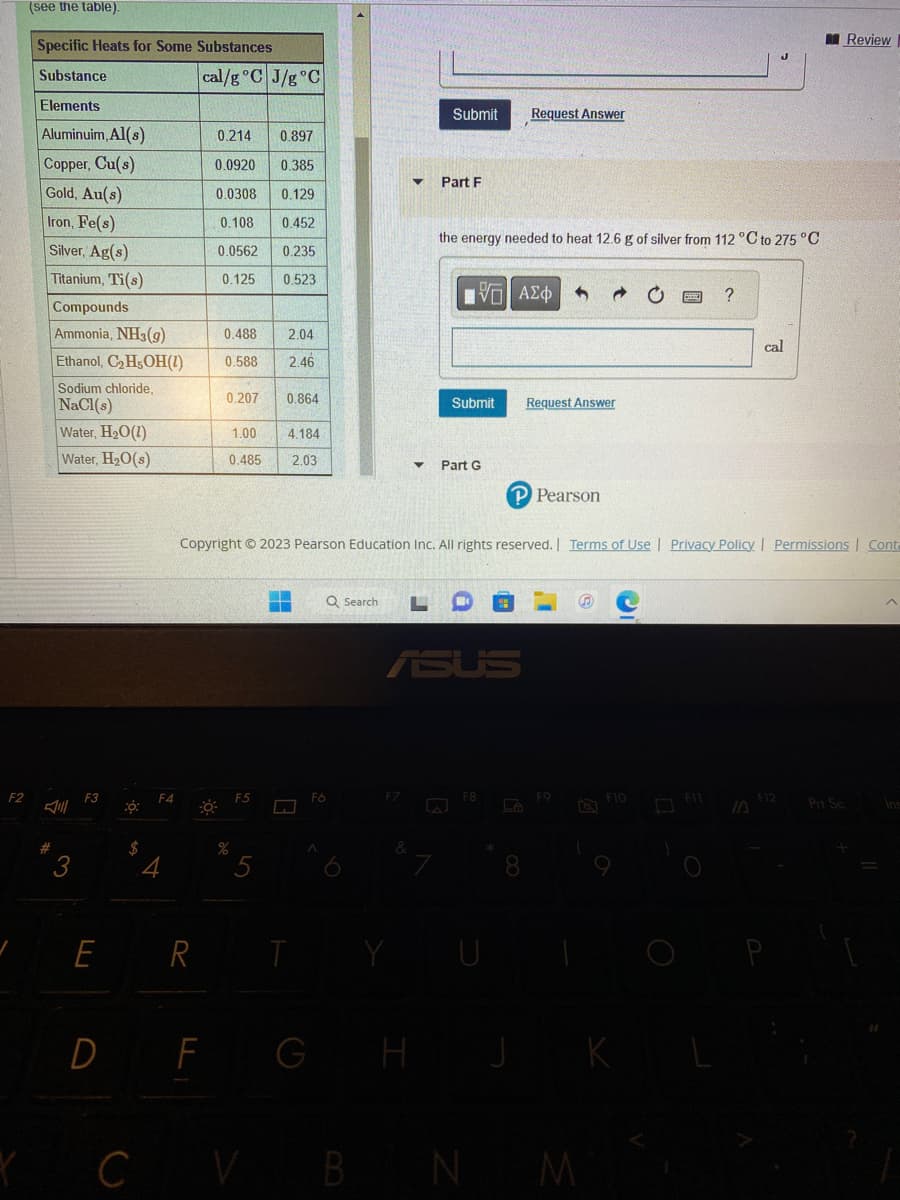
(see the table).
Specific Heats for Some Substances
Substance
Elements
Aluminuim, Al(s)
Copper, Cu(s)
Gold, Au(s)
Iron, Fe(s)
Silver, Ag(s)
Titanium, Ti(s)
#
Compounds
Ammonia, NH3(g)
Ethanol, C₂H5OH(1)
Sodium chloride,
NaCl(s)
Water, H₂O(1)
Water, H₂O(s)
3
F3
E
co:
$
F4
C
4
R
cal/g °C J/g °C
D F
0.214 0.897
0.0920 0.385
0.0308
0.129
0.108 0.452
0.0562
0.235
0.125 0.523
30:
0.488 2.04
0.588 2.46
0.207 0.864
1.00
0.485
%
5
4.184
2.03
V
77
Q Search
F6
G
Submit Request Answer
Copyright © 2023 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Conta
Part F
H
the energy needed to heat 12.6 g of silver from 112 °C to 275 °C
V—| ΑΣΦ 5
Submit
▼ Part G
ASUS
B N
P Pearson
Request Answer
8
FO
M
F10
?
F11
cal
F12
Review
Prt Sc
Ins
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning