Part II. Calculate the Atomic Mass 1. 0.337% 35 Ar; 0.063% 38A1; 99.60% 4ºA 2. 7.75% 68Ge; 20.52% 7ºGE; 27.43% 7²GE; 36.54% 74GE; 7.76% 76Ge 3. 0.089% 122TE; 2.46% 124TE; 5.48% 126TE; 91.97% 128TE
Part II. Calculate the Atomic Mass 1. 0.337% 35 Ar; 0.063% 38A1; 99.60% 4ºA 2. 7.75% 68Ge; 20.52% 7ºGE; 27.43% 7²GE; 36.54% 74GE; 7.76% 76Ge 3. 0.089% 122TE; 2.46% 124TE; 5.48% 126TE; 91.97% 128TE
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 96QRT: Disilane, Si2Hx, contains 90.28% silicon by mass. Calculate the value of x in this compound.
Related questions
Question
100%
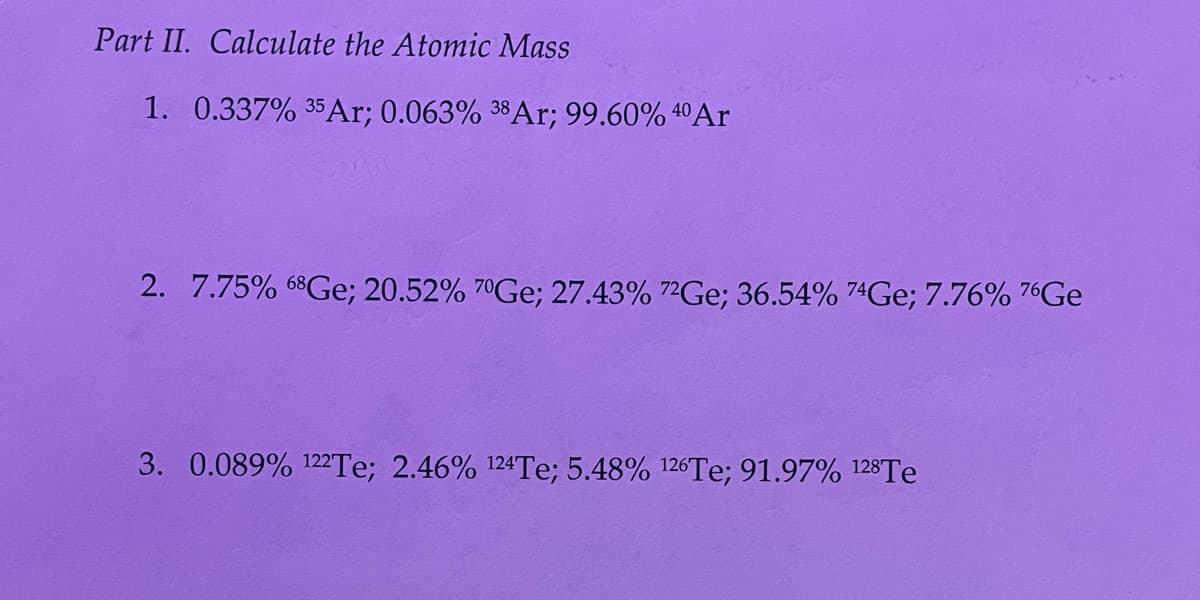
Transcribed Image Text:Part II. Calculate the Atomic Mass
1. 0.337% 35Ar; 0.063% 38A1; 99.60% 4ºAr
2. 7.75% 68Ge; 20.52% 7°GE; 27.43% 7²GE; 36.54% 74GE; 7.76% 76GE
3. 0.089% 122TE; 2.46% 124Te; 5.48% 126TE; 91.97% 128TE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning