2 Не H 1.008 4.003 5 6 B CN 0FNe 10.81 12.011 14.007 15.999 18.998 20.180 13 9 3 4 Li Be 7 8 10 6.94 9.012 12 15 11 Na Mg 22.990 24.305 14 Al 26.982 28.085 30.974 32.06 35.45 39.948 16 17 18 Si PS CI Ar Rank the following according to DECREASING number of S atoms: A. 31.0 g S8 B. 50.0 mL CS2 (density = 1.26 g/mL) C. 1.32 mol SF6 D. 4.67 × 1024 C5H4N4S molecules
2 Не H 1.008 4.003 5 6 B CN 0FNe 10.81 12.011 14.007 15.999 18.998 20.180 13 9 3 4 Li Be 7 8 10 6.94 9.012 12 15 11 Na Mg 22.990 24.305 14 Al 26.982 28.085 30.974 32.06 35.45 39.948 16 17 18 Si PS CI Ar Rank the following according to DECREASING number of S atoms: A. 31.0 g S8 B. 50.0 mL CS2 (density = 1.26 g/mL) C. 1.32 mol SF6 D. 4.67 × 1024 C5H4N4S molecules
Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.64E
Related questions
Question
Transcribed Image Text:se 10.81 12.011 14.007 15.999 18.998 20.180
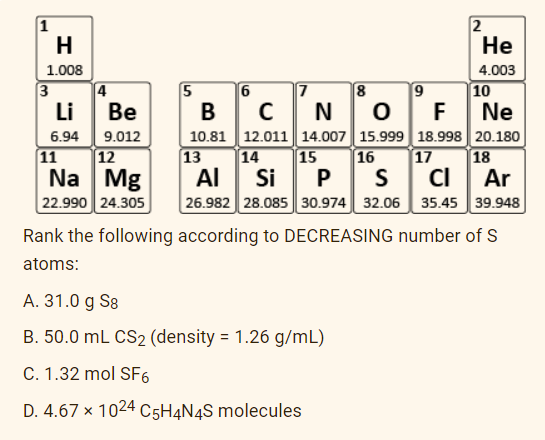
1
H
2
Не
1.008
4.003
3
4
5
19
8
CN OF
10.81 12.011 14.007 15.999 18.998 20.180
15
6
7
10
Ne
Li
Ве
В
6.94
9.012
11
17
18
12
Na Mg
22.990 24.305
13
16
Al Si P S
26.982 28.085 30.974 32.06 | 35.45 39.948
14
CI
Ar
Rank the following according to DECREASING number of S
atoms:
A. 31.0 g S8
B. 50.0 mL CS2 (density = 1.26 g/mL)
C. 1.32 mol SF6
D. 4.67 x 1024 C5H¼N4S molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div