Part of a series of radioactive elements is seen in table 1. Nuclide A changes to nuclide B with the emission of an a particle, nuclide B changes to nuclide C with the emission of a ß particle, etc. Using table 1, answer the following questions. Nuclide A B с D E F Atomic Number i. ii. 90 88 89 90 88 Nucleon Number 232 228 228 228 224 Table 1 a. State which particle is emitted when Nuclide C changes to nuclide D Nuclide D changes to nuclide E b. For nuclide F, state its i. Atomic number ii. Nucleon number c. Which nuclides are isotopes to each other? Radiation Emitted a B (t 25 Half Life 1.4 x 1010 years 6.7 years 6.1 years 1.9 years 3.6 days 52 seconds
Part of a series of radioactive elements is seen in table 1. Nuclide A changes to nuclide B with the emission of an a particle, nuclide B changes to nuclide C with the emission of a ß particle, etc. Using table 1, answer the following questions. Nuclide A B с D E F Atomic Number i. ii. 90 88 89 90 88 Nucleon Number 232 228 228 228 224 Table 1 a. State which particle is emitted when Nuclide C changes to nuclide D Nuclide D changes to nuclide E b. For nuclide F, state its i. Atomic number ii. Nucleon number c. Which nuclides are isotopes to each other? Radiation Emitted a B (t 25 Half Life 1.4 x 1010 years 6.7 years 6.1 years 1.9 years 3.6 days 52 seconds
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.59PAE
Related questions
Question
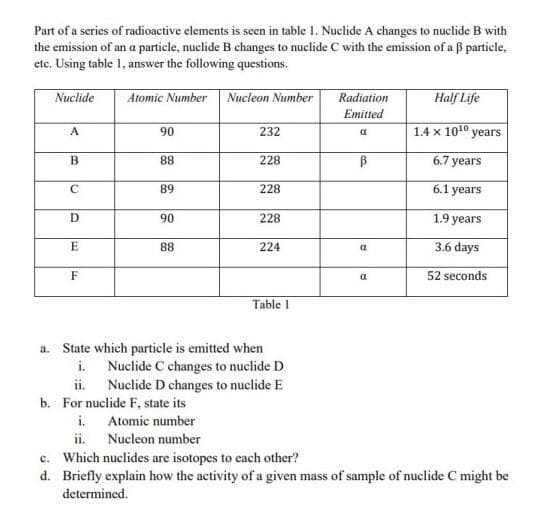
Transcribed Image Text:Part of a series of radioactive elements is seen in table 1. Nuclide A changes to nuclide B with
the emission of an a particle, nuclide B changes to nuclide C with the emission of a 3 particle,
etc. Using table 1, answer the following questions.
Atomic Number
Nuclide
A
B
C
D
E
F
i.
ii.
90
88
i.
ii.
89
90
88
b. For nuclide F, state its
Atomic number
Nucleon number
Nucleon Number
232
228
228
228
a. State which particle is emitted when
Nuclide C changes to nuclide D
Nuclide D changes to nuclide E
224
Table 1
Radiation
Emitted
a
В
a
α
Half Life
1.4 x 10¹0 years
6.7 years
6.1 years
1.9 years
3.6 days
52 seconds
c. Which nuclides are isotopes to each other?
d. Briefly explain how the activity of a given mass of sample of nuclide C might be
determined.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co