perature (Tg) is the temperature range where a polymer chain gains segmental bility of the polymer chains depends on (1) the presence of stiffening and flexibili rporated in the polymer backbone and (2) the presence of functional groups atta mer backbone. These functional groups attached to the polymer backbone is cll ps. For example, polyethylene terephthalate (PET) has a higher glass transition temp ethylene adipate. The terephthalic acid moiety in polyethylene terephthalate siderably an inflexible unit. Furthermore, the terephthalic acid moiety stiffens t kbone and restricts polymer chain mobility. On the other hand, the adipic acic ethylene adipate is flexible; thus, making the polymer backbone chains more freely ct of pendant groups in the glass transition temperature is very evident by comparin sition temperatures of low density polyethylene (LDPE) and polyvinyl alcohol (PVA). tively higher glass transition temperature than LDPE attributed to the presence of t p (-OH) which can form strong hydrogen bonds with other polymer chains. Wherea n polymer chains can only interact with each other via the much weaker London disper glass transition temperature of polymers also increase due to polymer backbone sy presence of crosslinks Adding additives such as plasticizers on the other hand de
perature (Tg) is the temperature range where a polymer chain gains segmental bility of the polymer chains depends on (1) the presence of stiffening and flexibili rporated in the polymer backbone and (2) the presence of functional groups atta mer backbone. These functional groups attached to the polymer backbone is cll ps. For example, polyethylene terephthalate (PET) has a higher glass transition temp ethylene adipate. The terephthalic acid moiety in polyethylene terephthalate siderably an inflexible unit. Furthermore, the terephthalic acid moiety stiffens t kbone and restricts polymer chain mobility. On the other hand, the adipic acic ethylene adipate is flexible; thus, making the polymer backbone chains more freely ct of pendant groups in the glass transition temperature is very evident by comparin sition temperatures of low density polyethylene (LDPE) and polyvinyl alcohol (PVA). tively higher glass transition temperature than LDPE attributed to the presence of t p (-OH) which can form strong hydrogen bonds with other polymer chains. Wherea n polymer chains can only interact with each other via the much weaker London disper glass transition temperature of polymers also increase due to polymer backbone sy presence of crosslinks Adding additives such as plasticizers on the other hand de
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.19E
Related questions
Question
I need a script/simple explanation of this lesson for my presentation. Thank you!
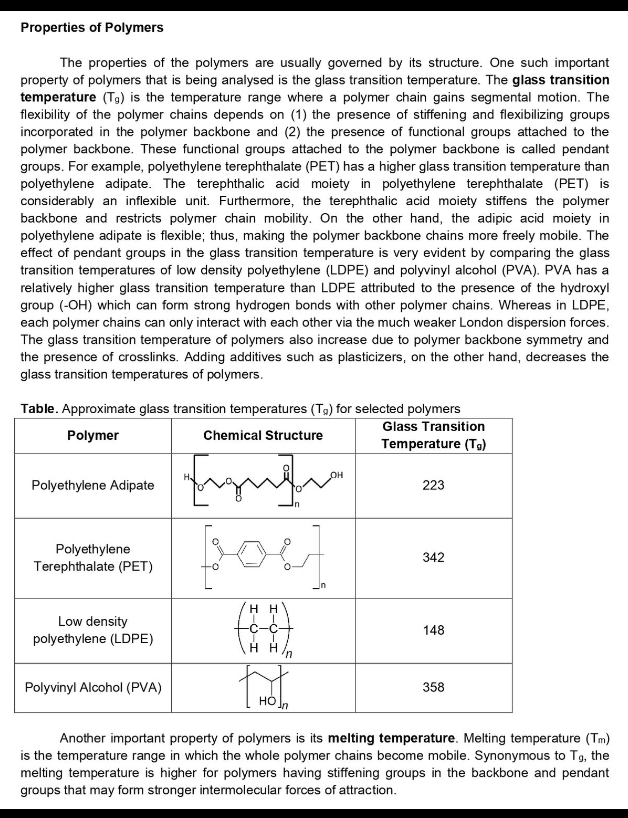
Transcribed Image Text:Properties of Polymers
The properties of the polymers are usually governed by its structure. One such important
property of polymers that is being analysed is the glass transition temperature. The glass transition
temperature (Tg) is the temperature range where a polymer chain gains segmental motion. The
flexibility of the polymer chains depends on (1) the presence of stiffening and flexibilizing groups
incorporated in the polymer backbone and (2) the presence of functional groups attached to the
polymer backbone. These functional groups attached to the polymer backbone is called pendant
groups. For example, polyethylene terephthalate (PET) has a higher glass transition temperature than
polyethylene adipate. The terephthalic acid moiety in polyethylene terephthalate (PET) is
considerably an inflexible unit. Furthermore, the terephthalic acid moiety stiffens the polymer
backbone and restricts polymer chain mobility. On the other hand, the adipic acid moiety in
polyethylene adipate is flexible; thus, making the polymer backbone chains more freely mobile. The
effect of pendant groups in the glass transition temperature is very evident by comparing the glass
transition temperatures of low density polyethylene (LDPE) and polyvinyl alcohol (PVA). PVA has a
relatively higher glass transition temperature than LDPE attributed to the presence of the hydroxyl
group (-OH) which can form strong hydrogen bonds with other polymer chains. Whereas in LDPE,
each polymer chains can only interact with each other via the much weaker London dispersion forces.
The glass transition temperature of polymers also increase due to polymer backbone symmetry and
the presence of crosslinks. Adding aditives such as plasticizers, on the other hand, decreases the
glass transition temperatures of polymers.
Table. Approximate glass transition temperatures (Tg) for selected polymers
Glass Transition
Temperature (Tg)
Polymer
Chemical Structure
он
Polyethylene Adipate
223
Polyethylene
Terephthalate (PET)
342
нн
Low density
-
148
polyethylene (LDPE)
H H
Polyvinyl Alcohol (PVA)
358
Но
Another important property of polymers is its melting temperature. Melting temperature (Tm)
is the temperature range in which the whole polymer chains become mobile. Synonymous to Tg, the
melting temperature is higher for polymers having stiffening groups in the backbone and pendant
groups that may form stronger intermolecular forces of attraction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,