Perform the following calculation: -0.0060 cm3 + 1.0042 cm3 What is the answer to the calculation above with the correct units and significant figures? O 1.0 –9 0.99820 0.9982 0.998 Questlon 9 Perform the following calculation: (-0.0030-o *275 °C)- 1.0042 x 27 °C x cm³ g °C+1.0042 -9 cm3 What is the answer to the calculation above with the correct units and significant figures? O 0.9960–9 O 1.0–9 cm3 0.996–9 0.99595 –9
Perform the following calculation: -0.0060 cm3 + 1.0042 cm3 What is the answer to the calculation above with the correct units and significant figures? O 1.0 –9 0.99820 0.9982 0.998 Questlon 9 Perform the following calculation: (-0.0030-o *275 °C)- 1.0042 x 27 °C x cm³ g °C+1.0042 -9 cm3 What is the answer to the calculation above with the correct units and significant figures? O 0.9960–9 O 1.0–9 cm3 0.996–9 0.99595 –9
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 73QAP: A Different civilization on a distant planet has developed a new temperature scale based on ethyl...
Related questions
Question
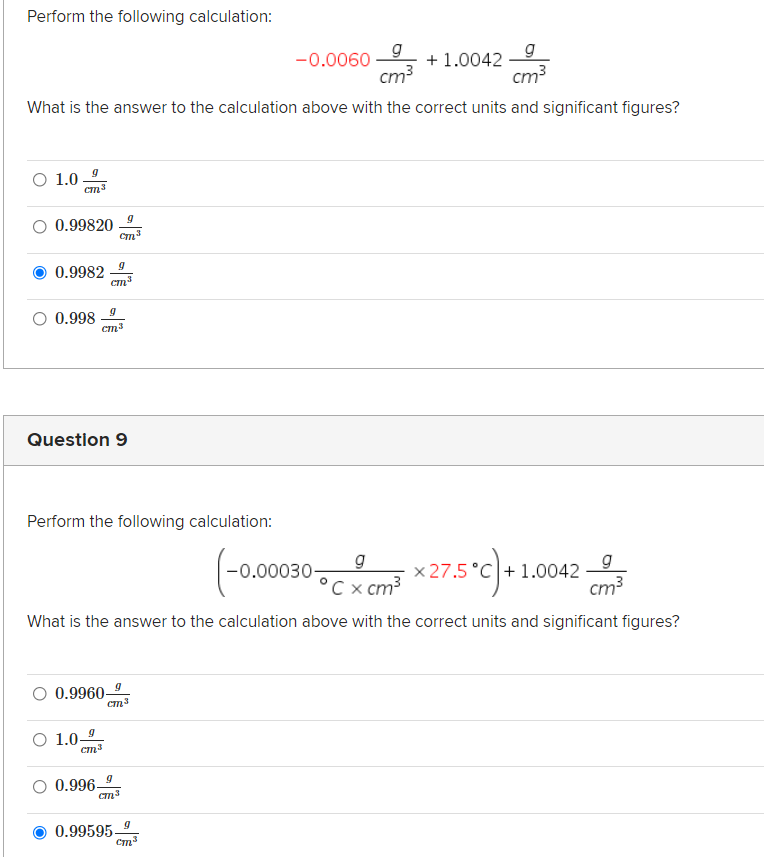
Transcribed Image Text:Perform the following calculation:
g
-0.0060
g
+ 1.0042
cm?
cm3
What is the answer to the calculation above with the correct units and significant figures?
O 1.0
O 0.99820
0.9982
Cm
O 0.998
cm3
Question 9
Perform the following calculation:
g
°C x cm?
*275°c)
g
x 27.5 °C + 1.0042
cm3
-0.00030-
What is the answer to the calculation above with the correct units and significant figures?
O 0.9960
O 1.0-
cm3
0.996-
0.99595-
Cm3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning