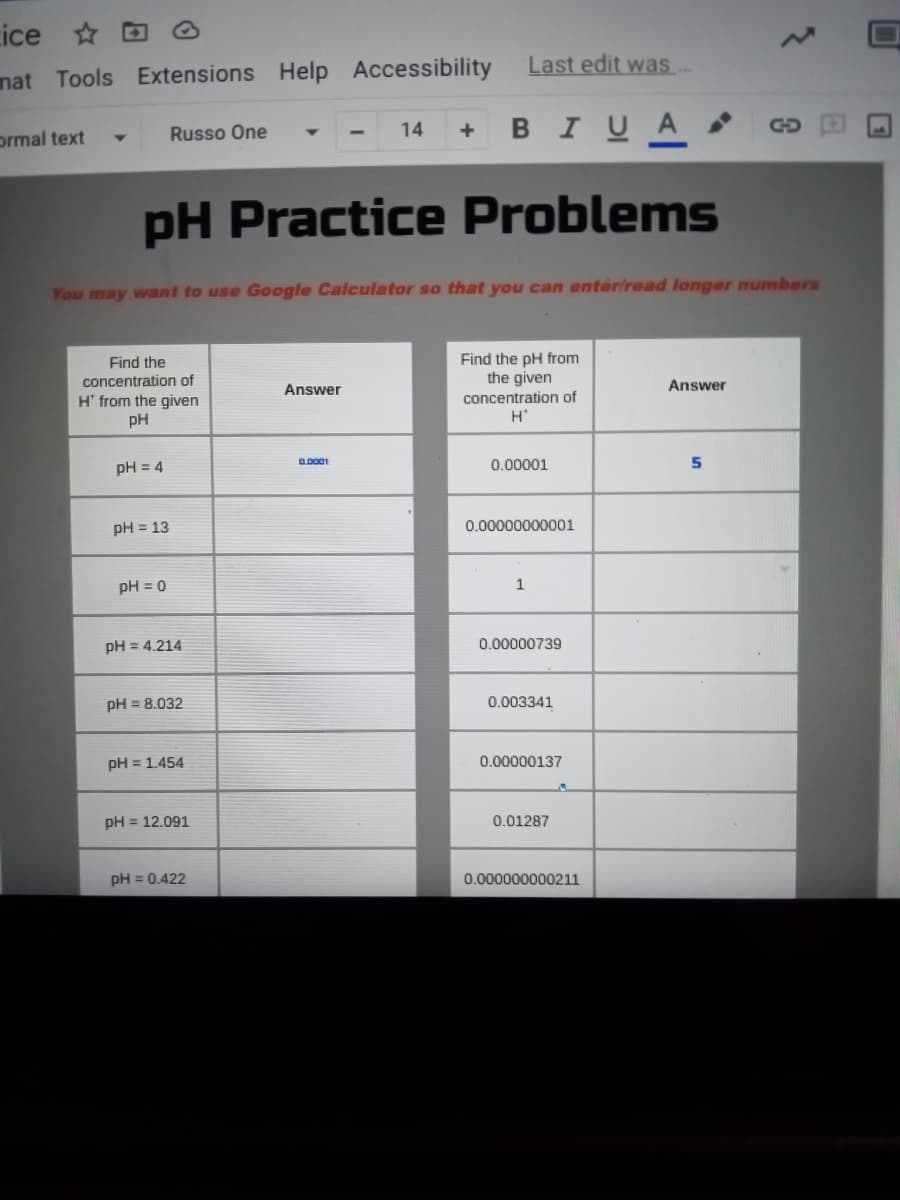
pH Practice Problems You may want to use Google Calculator so that you can enteriread longer numbers Find the concentration of Find the pH from the given concentration of Answer Answer H' from the given pH pH = 4 0.00001 pH = 13 0.00000000001 %3D pH = 0 pH = 4.214 0.00000739 %3D pH = 8.032 0.003341 pH = 1.454 0.00000137 pH = 12.091 0.01287 pH = 0.422 0.000000000211 5n
pH Practice Problems You may want to use Google Calculator so that you can enteriread longer numbers Find the concentration of Find the pH from the given concentration of Answer Answer H' from the given pH pH = 4 0.00001 pH = 13 0.00000000001 %3D pH = 0 pH = 4.214 0.00000739 %3D pH = 8.032 0.003341 pH = 1.454 0.00000137 pH = 12.091 0.01287 pH = 0.422 0.000000000211 5n
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 36QAP: There is a buffer system in blood H2PO4 HPO42 that helps keep the blood pH at 7.40. (K aH2PO4...
Related questions
Question
Transcribed Image Text:cice D ☺
Last edit was..
nat Tools Extensions Help Accessibility
в IUA
prmal text
Russo One
14
GD
pH Practice Problems
You may want to use Google Calculator so that you can enter/read longer numbers
Find the
concentration of
Find the pH from
the given
concentration of
Answer
Answer
H' from the given
pH
H'
pH = 4
0.00001
5
pH = 13
0.00000000001
pH = 0
pH = 4.214
0.00000739
pH = 8.032
0.003341
pH = 1.454
0.00000137
pH = 12.091
0.01287
pH = 0.422
0.000000000211
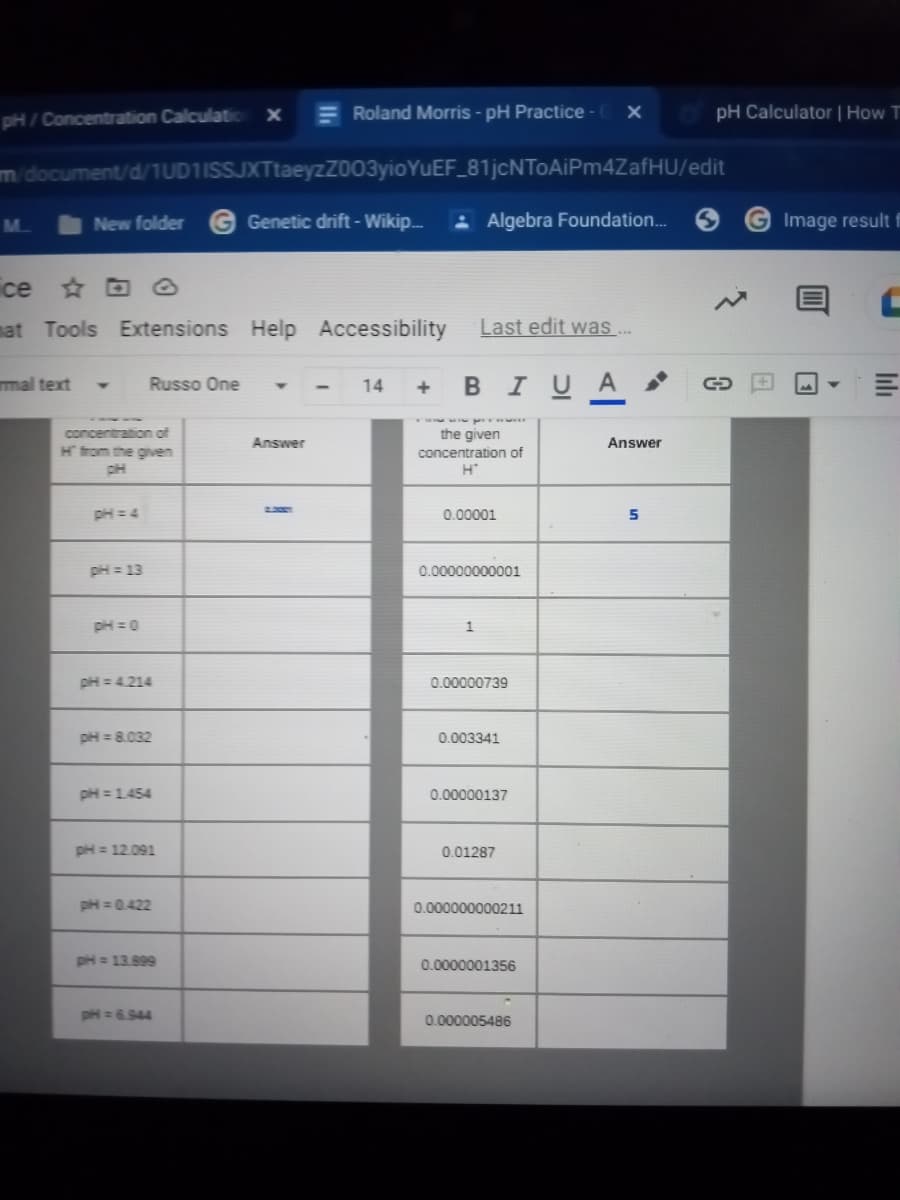
Transcribed Image Text:pH/ Concentration Calculatio
= Roland Morris - pH Practice - X
pH Calculator | How T
m/document/d/1UD1ISSJXTtaeyzZ003yioYuEF_81jcNToAiPm4ZafHU/edit
New folder
G Genetic drift - Wikip.
: Algebra Foundation..
Image result f
M.
ice * D ☺
mat Tools Extensions Help Accessibility
Last edit was ...
в IU A
mal text
Russo One
14
concentration of
H trom the given
the given
Answer
Answer
concentration of
pH = 4
2.
0.00001
5
pH = 13
0.00000000001
pH =0
pH = 4.214
0.00000739
pH = 8.032
0.003341
pH =1454
0.00000137
pH = 12.091
0.01287
pH = 0.422
0.000000000211
pH = 13.899
0.0000001356
pH = 6.944
0.000005486
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
