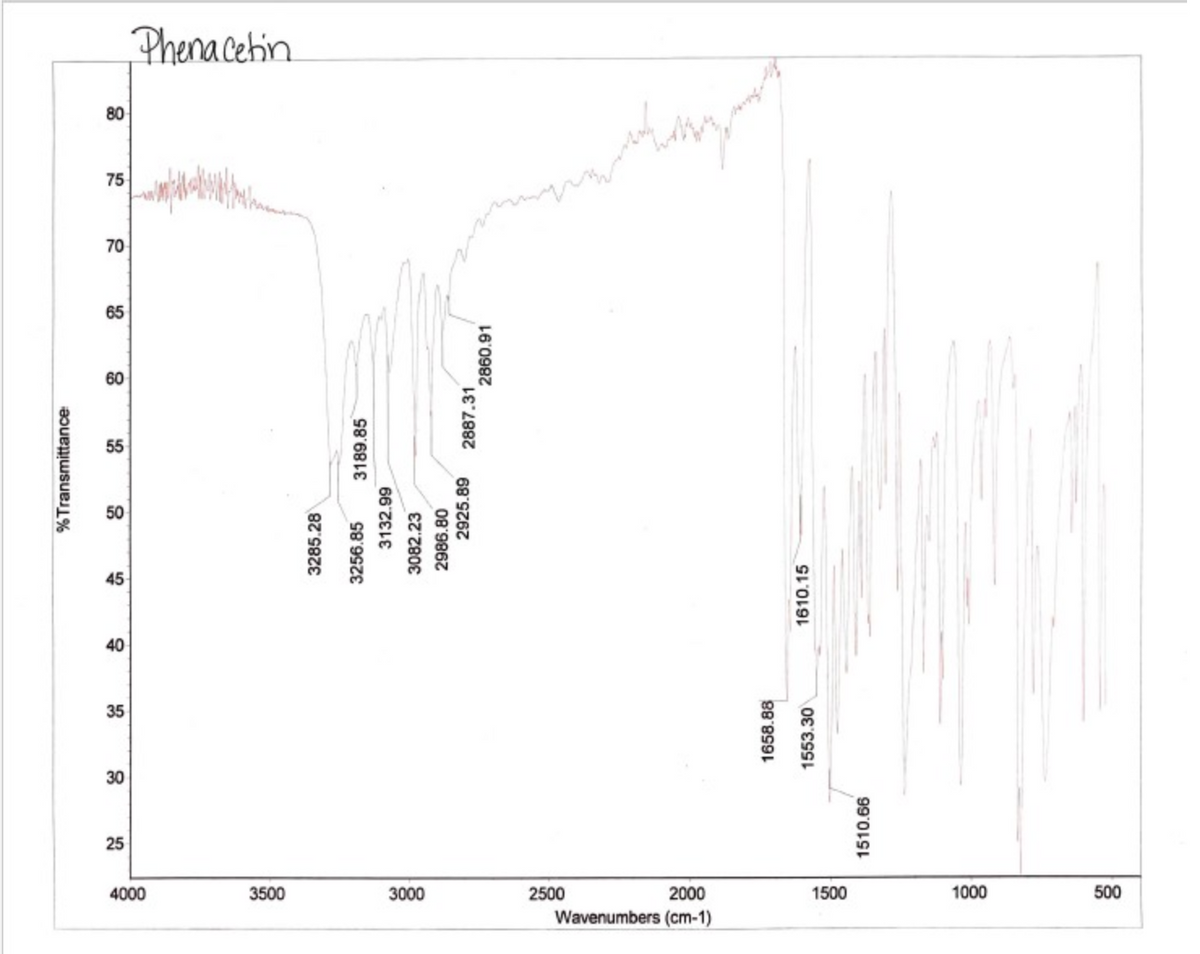
Phena Cetin 80 75 70 65 60 55 45 40 35 30 25 4000 3500 3000 2500 2000 1500 1000 500 Wavenumbers (cm-1) %Transmittance 3285.28 3256.85 3189.85 3132.99 3082.23 2986.80 2925.89 2887.31 2860.91 1658.88 1553.30 1610.15 1510.66
Can you answer this question please according to the prodecure and IR graph
Please talk and describe all the details in the IR graph and how pure our substance was accoring to the IR graph
1.063 g of p-acetamidophenol was measured out into a 50 mL round bottom flask along with 8 mL of 1M NaOH in ethanol and a stir bar. This mixture was heated at reflux for 15 min. It was allowed to cool and then 0.80 mL of bromoethane was added to the reaction. The mixture was then heated at reflux for another 15 min. After heating, the solution was poured onto a mixture of ice and water and further cooled on ice and then the solid was collected by vacuum filtration. This solid was then recrystallized from ethanol and water. The final mass of the purified phenacetin was 0.42250 g
Step by step
Solved in 2 steps with 1 images






