Phosphine is a colorless toxic gas. It is a hydride of phosphorus. The chemical formula of this compound is PH3. On the other hand, Silane is also a colorless, pyrophoric, toxic gas with a sharp, repulsive smell. It is also a hydride of silicone. The chemical formula of this compound is SiH4. According to the VSEPR model, provide comments, on the arrangement of electron pairs around PH3 and SİHA. Select one: O a. In each case there are a different number of electron pairs around the central atom, therefore different electron pairs arrangement around the central atom. Ob. Since both phosphorus and silicone are in the second period, and making hydride, therefore same electron pairs arrangement around the central atom. O c. Electron pairs arrangement around the central atom (different or the same), depending on the conditions leading to maximum repulsion and we don't have that information therefore can not comments. O d. In each case, the numbers of electron pairs around the central atom are same, therefore same electron pairs arrangement around the central atom. O e. In each case there are a different number of atoms around the central atom, therefore different electron pairs arrangement around the central atom.
Phosphine is a colorless toxic gas. It is a hydride of phosphorus. The chemical formula of this compound is PH3. On the other hand, Silane is also a colorless, pyrophoric, toxic gas with a sharp, repulsive smell. It is also a hydride of silicone. The chemical formula of this compound is SiH4. According to the VSEPR model, provide comments, on the arrangement of electron pairs around PH3 and SİHA. Select one: O a. In each case there are a different number of electron pairs around the central atom, therefore different electron pairs arrangement around the central atom. Ob. Since both phosphorus and silicone are in the second period, and making hydride, therefore same electron pairs arrangement around the central atom. O c. Electron pairs arrangement around the central atom (different or the same), depending on the conditions leading to maximum repulsion and we don't have that information therefore can not comments. O d. In each case, the numbers of electron pairs around the central atom are same, therefore same electron pairs arrangement around the central atom. O e. In each case there are a different number of atoms around the central atom, therefore different electron pairs arrangement around the central atom.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter20: The Representative Elements
Section: Chapter Questions
Problem 10RQ
Related questions
Question
With steps pls
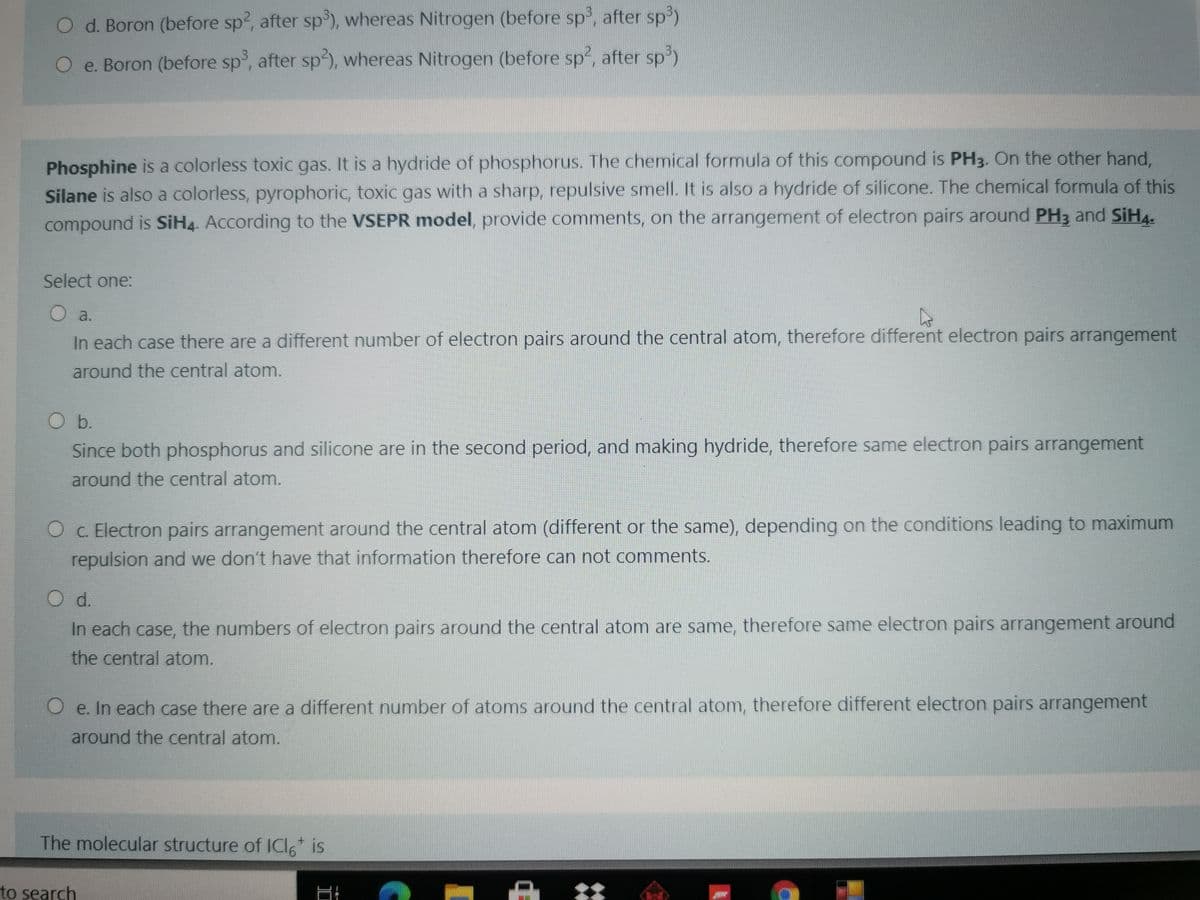
Transcribed Image Text:O d. Boron (before sp, after sp'), whereas Nitrogen (before sp, after sp3)
O e. Boron (before sp, after sp), whereas Nitrogen (before sp2, after sp)
Phosphine is a colorless toxic gas. It is a hydride of phosphorus. The chemical formula of this compound is PH3. On the other hand,
Silane is also a colorless, pyrophoric, toxic gas with a sharp, repulsive smell. It is also a hydride of silicone. The chemical formula of this
compound is SiH4. According to the VSEPR model, provide comments, on the arrangement of electron pairs around PH3 and SIH4-
Select one:
O a.
In each case there are a different number of electron pairs around the central atom, therefore different electron pairs arrangement
around the central atom.
Ob.
Since both phosphorus and silicone are in the second period, and making hydride, therefore same electron pairs arrangement
around the central atom.
O c. Electron pairs arrangement around the central atom (different or the same), depending on the conditions leading to maximum
repulsion and we don't have that information therefore can not comments.
O d.
In each case, the numbers of electron pairs around the central atom are same, therefore same electron pairs arrangement around
the central atom.
O e. In each case there are a different number of atoms around the central atom, therefore different electron pairs arrangement
around the central atom.
The molecular structure of IClo is
to search
|品 は
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
