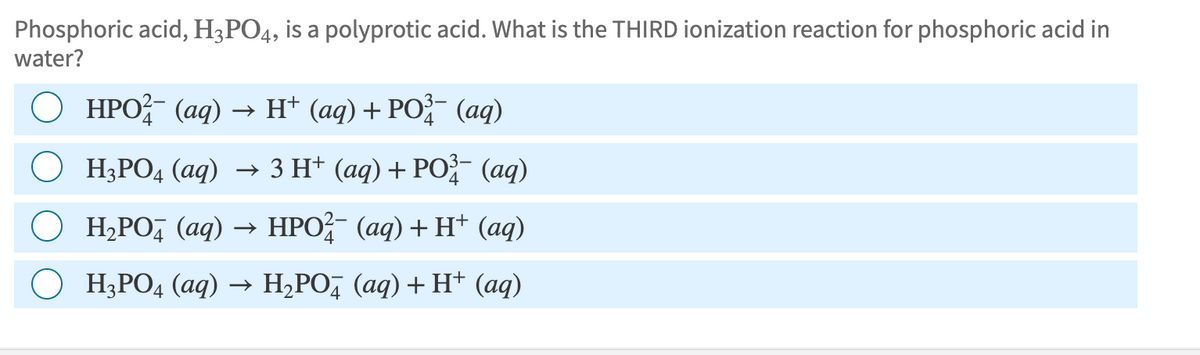
Phosphoric acid, H3PO4, is a polyprotic acid. What is the THIRD ionization reaction for phosphoric acid in water? HPO (aq) → H+ (aq) + PO³ (aq) ○ H3PO4 (aq) → 3 H+ (aq) + PO¾- (aq) 4 H₂PO (aq) → HPO²¼¯ (aq) + H+ (aq) H3PO4 (aq) → H₂PO (aq) + H+ (aq)
Q: Question 28 Predict the FINAL (?) product for each of the following reaction or synthetic chain: Mgl…
A: The sequence of reactions shown suggests the formation of a Grignard reagent followed by a reaction…
Q: Draw the major product of this reaction. Ignore inorganic byproducts. Taken O 1. CH3MgBr 2.…
A: Step 1:
Q: 2. Standard ... M 3. AS surrou*** 1req 4. AG° = AH°... M 5. AG: Pre... 1req 6. AG: Enthal... M 7. AG…
A: ΔGrxn = ΔHrxn - TΔS 2 NO(g) + O₂(g) → 2 NO₂(g) Standard enthalpies of formation: (This is a constant…
Q: 5(a) The Joule-Thomson experiment (JTE) is an example of an isenthalpic process i) Define an…
A: The objective of the first part of the question is to define an isenthalpic process, show that the…
Q: In a major human artery with an internal diameter of 5 mm, the flow of blood, averaged over the…
A:
Q: and 18 [References] 1 pt 2 pts 1 pt 2 pts 1 pt 2 pts Strontium hydroxide, Sr(OH)2, dissolves in…
A: Step 1:The dissociation equation for strontium hydroxide (Sr(OH)₂) dissolving in water is: Sr(OH)₂…
Q: 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g)Using the standard thermodynamic data in the tables linked above,…
A: - ΔG° is the standard Gibbs free energy change for the reaction.- R is the universal gas constant,…
Q: 3. What changes occur to the atomic number and mass of a nucleus during each of the following decay…
A: The symbol of an element is expressed as, A XZ Where, X = the atomic symbol of the element (like…
Q: Consider three hypothetical solids:AX,AX2 and AX3 (each X forms X). Each of these solids has the…
A: Solution : Solubility is defined as the maximum amount of solute dissolved in an aqueous…
Q: Macmillan Learning An 80.0 g sample of a gas was heated from 25 °C to 225 °C. During this process,…
A: The objective of the question is to calculate the specific heat of the gas. The specific heat of a…
Q: Draw the starting reactant that would produce this alcohol when treated with this reagent. Ignore…
A: Thank you,Please rate my response.
Q: MISSED THIS? Watch KCV: Solubility and The Solubility Product Constant, IWE: Calculating Molar…
A: CuCl <=> Cu+ + Cl-Mass of CuCl in 1 L (1000 mL) of solution = 1000/100 x 3.76= 37.6 mg =…
Q: Question 24 Predict the FINAL (?) product for each of the following reaction or synthetic chain: ?…
A: Approach to solving the question: Detailed explanation: Examples: Key references:
Q: Determine the temperature of a reaction if K = 1.20 x 10⁻⁶ when ∆G° = +26.90 kJ/mol.
A: The objective of this question is to calculate the temperature of a reaction given the standard…
Q: Establish the reaction and propose a mechanism for the reaction of benzene with bromine catalyzed…
A:
Q: How many grams of gold (Au)are there in 16.13 moles of Au? Round your answer to 4 significant…
A: Approach to solving the question: Detailed explanation: Examples: Key references:
Q: - Aktiv Chemistry ← → C M Gmail b Answered: Draw the major pro × | + app.aktiv.com ☑ ੩ ॥ YouTube…
A: Thank you.
Q: Problem 34 of 50 Submit Draw the product that could be formed when 1,3-butadiene reacts with…
A: Step 1:Mechanism Diels-Alder Reaction:It's a pericyclic reaction, meaning all bond breaking and…
Q: Predict the products of the following reaction. If no reaction will occur, use the NO REACTION…
A: Step 1:When solid strontium (Sr) reacts with hydrobromic acid (HBr), a single displacement reaction…
Q: 2. When an anhydrous solution of CrCl₂ in EtOH was treated with ligand 1 (below), a precipitate of…
A: (a) Analytical Data Interpretation and Possible Structures for X and YGiven the analytical and…
Q: Please don't provide handwritten solution
A: Approach to solving the question: Detailed explanation: Examples: Key references:
Q: What is the pH of a solution made by dissolving 4.61 grams of calcium fluoride in enough water to…
A: Calculate the moles of CaF2: The molar mass of CaF2 is approximately 78.08 g/mol. So, the moles of…
Q: Sodium hydroxide is a substance that causes severe burns. It is used in many educational laboratory…
A: The objective of the question is to identify the correct statements about the nature of sodium…
Q: Is there a minimum standard reduction potential that the half- reaction used at the cathode of this…
A: Approach to solving the question: there is a minimum standard reduction potential, but there is no…
Q: S.s.s.g.g.a.a.r
A: The objective of the question is to write the Henderson-Hasselbalch equation for a propanoic acid…
Q: Imagine the main chain of a protein bends back on itself, so that two amino acid residues R₁ and R2…
A: Step 1.Step 2. Answer:
Q: Below is the resolved absorption spectrum of HBr for the vibrational transition v = 0 → V = 1;…
A: Our usual method of determining the effective spring constant and bond length from the resolved…
Q: Question 7 Devise a synthesis for the dicarbonyl-containing compound 1 from 2. The available…
A: The objective of the question is to devise a synthesis for a dicarbonyl-containing compound from a…
Q: ↓ Draw the major product of this reaction. Include stereochemistry if applicable. Ignore byproducts.…
A: Step 1: Step 2: Step 3: Step 4:
Q: Give the IUPAC name of the molecule.
A: Step 1:name of the molecule is "3-tert-butylcyclopentane-1-carboxylic acid"Explanation 1. Select…
Q: In an attempt to synthesize compound C through a two-step process, a chemist discovered after…
A: Step 1:• In this reaction, Firstly base abstract the acidic proton to forms enolate ion which…
Q: Can you please set up the ice table?
A: The given buffer solution consists of a weak base,C6H5NH2 and its salt ,C6H5NH3Cl.C6H5NH2 is a weak…
Q: The first enzyme to rise in acute pancreatitis is ________________, and the enzyme which stays…
A: The objective of the question is to identify the enzymes that are affected during acute…
Q: 5. Provide the complete IUPAC name for the following molecule. Br O
A: Step 1: Determine the different functional groups in the structure. In this structure, there are…
Q: Predict the major product of the following process. 1) LDA i 2) 130+ OH im OH ii м ཚིག་དྲིས་ཏང་ ? O-
A:
Q: Find the pH of the following solutions. First write the balanced equilibrium equation of this salt…
A:
Q: mechanism reaction of 1, 3butadiene and maleic anhydride
A: The objective of this question is to understand the mechanism of reaction between 1,3-butadiene and…
Q: Please answer in tipping format
A: F)The reaction of CH3-CH2-CO-OH (propionic acid) with PCl3 and 2(CH2)2NH (diethylamine) typically…
Q: 2. Treatment of the ketone shown below with ZnBH4 in diethyl ether provides a 94:6 mixture of B and…
A: Given that ZnBH4 reduces the ketone to give a 94:6 mixture of B and C, we can infer that B is the…
Q: To obtain 13.0 g of sucrose (MM = 342) from a solution labeled 6.3% C12H22O11 by mass, we would need…
A: The objective of this question is to find out the amount of solution needed to obtain a specific…
Q: All are metabolic pathways for glucose catabolism (metabolism) EXCEPT: Question 70 options:…
A: The question is asking us to identify which of the given options is not a metabolic pathway for…
Q: :$;$;$;:$:&;&;&&;&
A:
Q: O Chemical Reactions Using molarity to find solute moles and solution volume 0/5 Bish A chemist adds…
A: To find the millimoles of barium chlorate (Ba(ClO3)2) added, we can follow these steps:Convert the…
Q: 9 1/1 point For which salt in each of the following groups will the solubility increase at lower pH?…
A: Step 1: Step 2:Step 3: Step 4:
Q: Explain how a mercury barometer works.
A: The objective of this question is to understand the working principle of a mercury barometer, which…
Q: High serum total protein with high levels of both albumin and globulins is usually seen in…
A: In Waldenström's macroglobulinemia:1. Malignant B-lymphocytes proliferate and overproduce monoclonal…
Q: Question 12 Choose the correct chemical structure for every single acronym used below: Bnl ? A B C D…
A: Option c: This option is correct
Q: For the electrochemical cell represented by this shorthand notation, which statement below is true?…
A: The objective of the question is to identify the correct statement about the electrochemical cell…
Q: In the following acid-base equilibria of weak acids in water, label the acid (A), the base (B), the…
A: The objective of the question is to identify the acid, base, conjugate acid, and conjugate base in…
Q: a. b. C. CH,CH-CH-CH-CH₂OH CH,CHCH,CHCH,CH3 CH₂CHCH CHCH CH₂ CH3 OH OH Br d. OH CH,CHCH2CH3 OH f.…
A: (a) 2-Pentanol:'Pent' indicates a five-carbon chain.'anol' indicates the presence of an alcohol…
Unlock instant AI solutions
Tap the button
to generate a solution
Click the button to generate
a solution
- The pH of an aqueous solution of 0.1690 M hydrosulfuric acid, H₂S (aq), isThe pH of an aqueous solution of 0.176 M tellurous acid, H2TeO3 (aq), isAssume you titrated to the endpoint 0.10 moles of HA (weak, monoprotic acid) with a solution of sodium hydroxide, NaOH(aq), of unknown concentration. The initial volume of NaOH(aq) in the buret was 5.00 mL, and the final volume of NaOH(aq) in the buret was 45.00 mL. What is the concentration, in molarity, of the NaOH solution used?
- The pH of an aqueous solution of 0.137 M sodium hypochlorite, NaClO (aq), is ________________ This solution is: ________ (ACIDIC, BASIC, OR NEUTRAL?)If weak acids ionize only a few percent in aqueous solution, why is it possible to fully neutralize a weak acid by reacting it with the stoichiometric equivalent of sodium hydroxide solution, NaOH(aq)?To measure the amount of calcium carbonate CaCO3 in a seashell, an analytical chemist crushes a 4.500g sample of the shell to a fine powder and titrates it to the endpoint with 172.mL of 0.380M hydrogen chloride HCl solution. The balanced chemical equation for the reaction is: →+2HClaq CO2−3aq + H2CO3aq 2Cl−aq What kind of reaction is this? Percipitation, acid-base, or redox Calculate the mass percent of CaCO3 in the sample. Be sure your answer has the correct number of significant digits.
- When acidulated water (dil.H2SO2solution) is electrolysed, will the pH of the solution be affected? Justify your answer.Phosphoric acid, H3PO4 (aq), is found in soda. Write the balanced chemical equation for the neutralization of phosphoric acid with NaOH.Complete and balance each of the following equations for acid-base reactions. H2SO4(aq)+Ca(OH)2(aq)→H2SO4(aq)+Ca(OH)2(aq)→ Express your answer as a chemical equation. Identify all of the phases in your answer.
- Assuming the base completely dissociates in water, what is the pH of a 5.70 g/L solution of Ba(OH) 2 (aq) ?Balanced equation for: AgNO3(aq) + NaOH(aq)A student prepares a dilute solution of sodium hydroxide, NaOH (aq), starting with 6 M sodium hydroxide. She then titrates a 1.372 g sample of KHP with the dilute sodium hydroxide solution, NaOH (aq), to a phenolphthalein end point. 1. If the titration required 21.84 mL of sodium hydroxide, NaOH (aq), calculate the molar concentration of the sodium hydroxide solution, NaOH (aq). (Remember that KHP is potassium hydrogen phthalate, KHC8H4O4, NOT potassium hydrogen phosphorus!) 2.The student uses the same sodium hydroxide to titrate 10.00 mL of vinegar to a phenolphthalein end point. If the titration required 27.48 mL of sodium hydroxide, NaOH (aq), calculate the molar concentration of acetic acid, HC2H3O2 (aq), in the vinegar. 3. Calculate the mass percent of acetic acid, HC2H3O2 (aq), in the vinegar using the molar concentration for acetic acid, HC2H3O2 (aq), determined in part b and assuming the density of the solution is 1.01 g/mL.

