What is the oxidation number of the monoatomic ions of the following elements? Drag each item to the appropriate bin. Reset Help sFLi Ca Na N Br AI o Mg H +3 +2 +1 -1 -2
What is the oxidation number of the monoatomic ions of the following elements? Drag each item to the appropriate bin. Reset Help sFLi Ca Na N Br AI o Mg H +3 +2 +1 -1 -2
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 35E: Write balanced chemical equations for the reactions used to prepare each of the following compounds...
Related questions
Question
PLEASE ANSWE.R BE.LOW
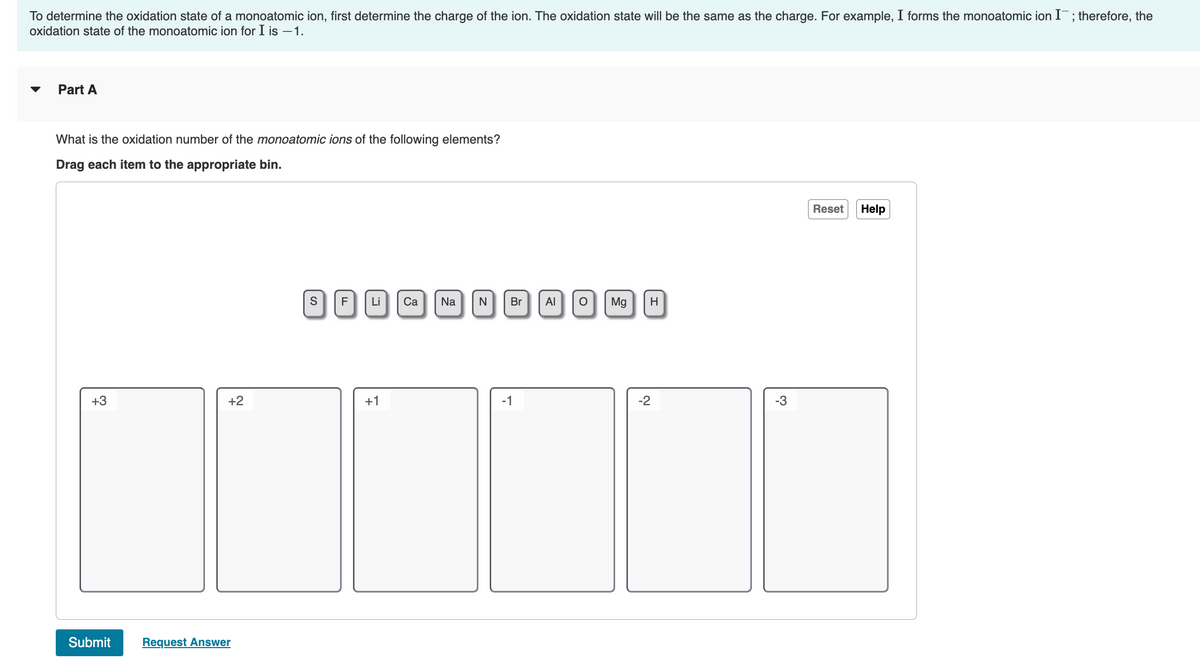
Transcribed Image Text:To determine the oxidation state of a monoatomic ion, first determine the charge of the ion. The oxidation state will be the same as the charge. For example, I forms the monoatomic ion I¯; therefore, the
oxidation state of the monoatomic ion for I is –1.
Part A
What is the oxidation number of the monoatomic ions of the following elements?
Drag each item to the appropriate bin.
Reset
Help
F
Li
Са
Na
Br
Al
Mg
H
+3
+2
+1
-1
-2
-3
Submit
Request Answer
|이

Transcribed Image Text:Learning Goal:
To assign oxidation states to elements in monoatomic ions, polyatomic ions, and neutral compounds.
An oxidation state (also called an oxidation number) is similar to a charge and allows you to keep track of electron transfer in oxidation-reduction reactions. Some guidelines for assigning oxidation states are
described here:
1. The oxidation state of an atom in a free element is 0. Examples of free elements include Mg, Cl2 , and N2.
2. The oxidation state of a monoatomic ion is equal to its charge. Examples of monoatomic ions include Mg²+ and Cl-, the individual ions present in the ionic compound MgCl2 .
3. The sum of the oxidation states of all atoms is
• for a neutral molecule or formula unit: zero; and
• for an ion: the charge of the ion.
4. In compounds,
• group 1A metals have an oxidation state of +1, and
group 2A metals have an oxidation state of +2.
5. In compounds, nonmetals are assigned oxidation states according to the following hierarchical table. Entries at the top of the table have priority over entries at the bottom.
Nonmetal Oxidation state
fluorine
-1
hydrogen
+1
охудen
-2
group 7A
-1
group 6A
-2
group 5A
-3
The oxidation states of monoatomic ions
To determine the oxidation state of a monoatomic ion, first determine the charge of the ion. The oxidation state will be the same as the charge. For example, I forms the monoatomic ion I¯; therefore, the
oxidation state of the monoatomic ion for I is –1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning