20. Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations: inol on (a) Mg(s) + NiCl2(aq) MgCl2(aq) + Ni(s) > (b) PCI3(1) + Cl2(g) PCI5(s)
20. Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations: inol on (a) Mg(s) + NiCl2(aq) MgCl2(aq) + Ni(s) > (b) PCI3(1) + Cl2(g) PCI5(s)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter6: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 5ALQ
Related questions
Question
100%
(For question 20 letter A) Would the reactant Cl2 which has a -1 charge and the product Cl2 also has a -1 charge; does that make it oxidation or reduced? Thank you!
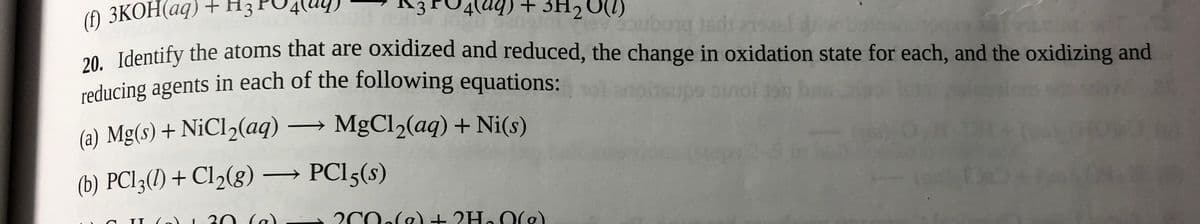
Transcribed Image Text:reducing agents in each of the following equations:
+ Нз
4) + 3H2O) ng leds anl d
() ЗКОН(ад)
20. Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and
(a) Mg(s) + N¡CI2(aq) → MgCl2(aq) + Ni(s)
(b) PCI3(1) + Cl2(g) → PCI5(s)
30 (a)
Expert Solution
Step 1
You have a good question
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning