Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 57QAP: Calcium ions in blood trigger clotting. To prevent that in donated blood, sodium oxalate, Na2C2O4,...
Related questions
Question
please answer 2nd quesion
when a nonvolatile solute dissolves in water to form a solution?
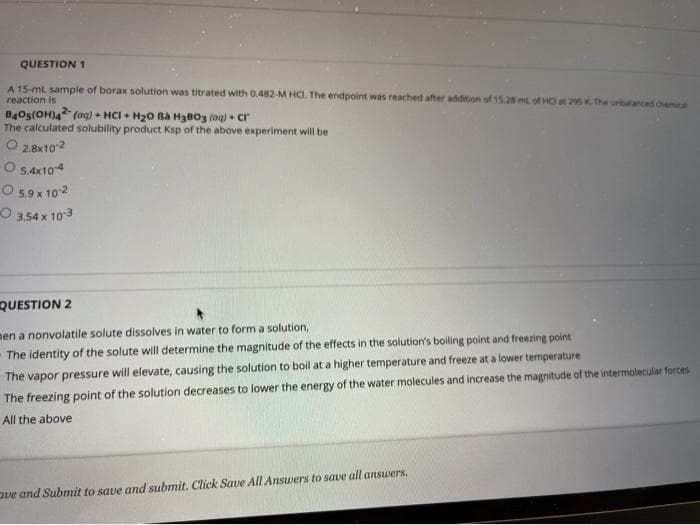
Transcribed Image Text:QUESTION 1
A 15-ml sample of borax solution was titrated with 0.482-M HCI. The endpoint was reached after addition of 15.28 mL of HC at 295 K. The urbalanced chemE
reaction is
B4Os(OH)4 (oaq) + HCI + H20 Ba H3BO3 (og) + cr
The calculated solubility product Ksp of the above experiment will be
O 2.8x102
O 54x104
O 5,9 x 102
O 3.54 x 10 3
QUESTION 2
nen a nonvolatile solute dissolves in water to form a solution,
The vapor pressure will elevate, causing the solution to boil at a higher temperature and freeze at a lower temperature
The freezing point of the solution decreases to lower the energy of the water molecules and increase the magnitude of the intermolecular forces
The identity of the solute will determine the magnitude of the effects in the solution's boiling point and freezing point
All the above
aue and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning