Please calculate the missing amounts in the table using the provided information. Reagent Table: Compound Anthracene Maleic Anhydride Molecular Weight (g/mol) 178.23 g/mol 98.06 g/mol Density (g/mL) N/A N/A Amount (g) mmol Equivalents 0.200 calculate calculate calculate Place 0.200 grams of anthracene and an equal molar amount of maleic anhydride in 5 mL of xylenes in a 10-mL round-bottom flask with a magnetic stir bar. 1 1
Please calculate the missing amounts in the table using the provided information. Reagent Table: Compound Anthracene Maleic Anhydride Molecular Weight (g/mol) 178.23 g/mol 98.06 g/mol Density (g/mL) N/A N/A Amount (g) mmol Equivalents 0.200 calculate calculate calculate Place 0.200 grams of anthracene and an equal molar amount of maleic anhydride in 5 mL of xylenes in a 10-mL round-bottom flask with a magnetic stir bar. 1 1
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
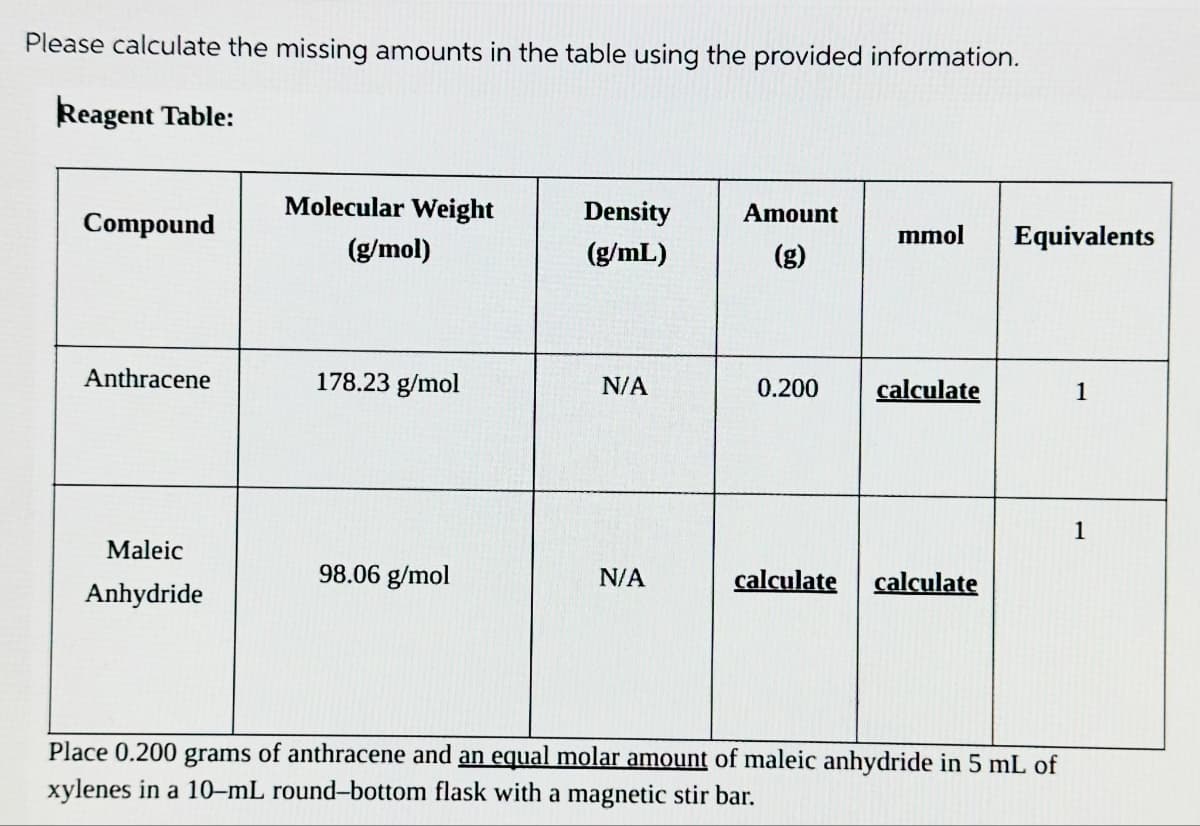
Transcribed Image Text:Please calculate the missing amounts in the table using the provided information.
Reagent Table:
Compound
Anthracene
Maleic
Anhydride
Molecular Weight
(g/mol)
178.23 g/mol
98.06 g/mol
Density
(g/mL)
N/A
N/A
Amount
(g)
mmol Equivalents
0.200 calculate
calculate calculate
Place 0.200 grams of anthracene and an equal molar amount of maleic anhydride in 5 mL of
xylenes in a 10-mL round-bottom flask with a magnetic stir bar.
1
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning