Please use the values in the resources listed below instead of the textbook values. Calculate the concentration (in M) of Ag* when AgIO, just begins to precipitate from a solution that is 0.0325 M in IO,. (K = 3.17×10-8) %3D 4.0 M.
Please use the values in the resources listed below instead of the textbook values. Calculate the concentration (in M) of Ag* when AgIO, just begins to precipitate from a solution that is 0.0325 M in IO,. (K = 3.17×10-8) %3D 4.0 M.
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.16QAP
Related questions
Question
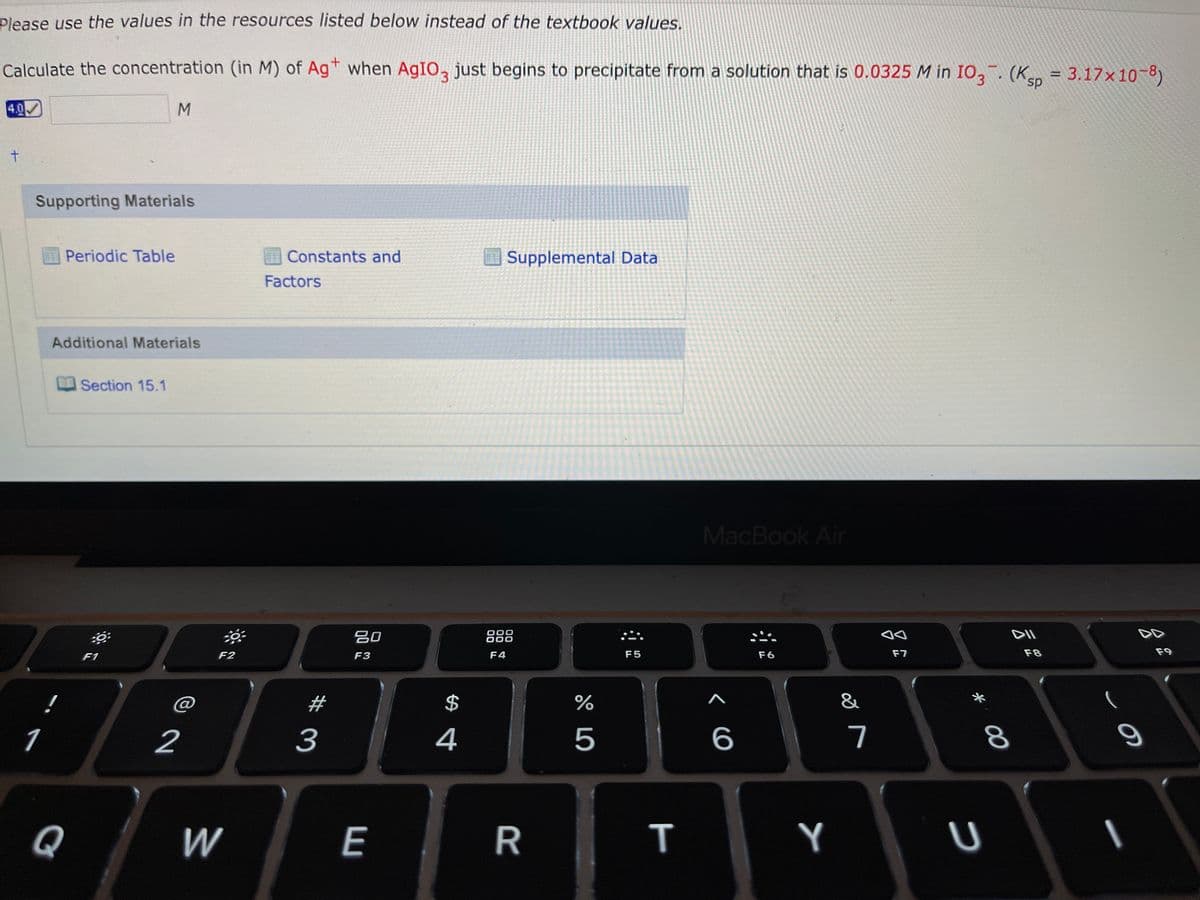
Transcribed Image Text:Please use the values in the resources listed below instead of the textbook values.
Calculate the concentration (in M) of Agt when AGIO, just begins to precipitate from a solution that is 0.0325 M in IO,. (Ksp = 3.17×10-8)
%D
4.0
Supporting Materials
Periodic Table
Constants and
Supplemental Data
Factors
Additional Materials
Section 15.1
MacBook Air
吕0
888
F1
F2
F3
F4
F5
F6
F7
F8
F9
@
2$
&
1
2
3
4
5
7
Q
W
E
R
Y
00
T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning