Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
Please help work this out using the conversion factor!
Transcribed Image Text:P Perusall
Chem101
G A solution of citric acid (H:CeHsC
+
Ô https://app.101edu.co
Not syncing
Question 13 of 21
Done
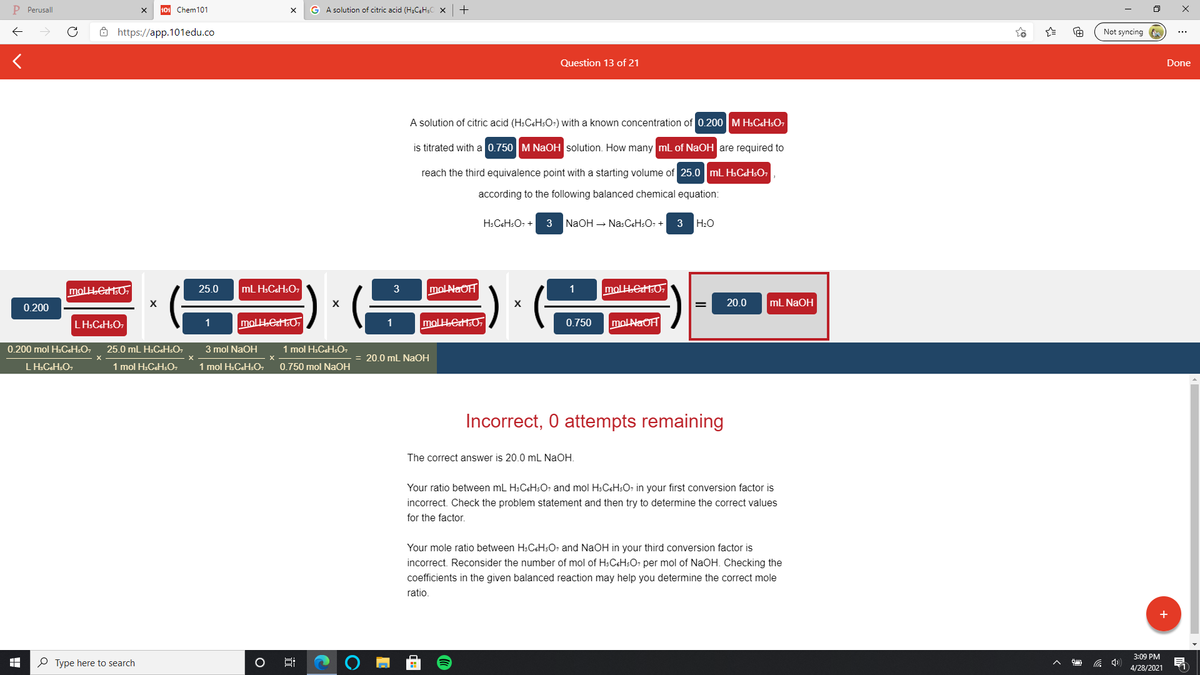
A solution of citric acid (H:CSH:O-) with a known concentration of 0.200 M HsCsHsO7
is titrated with a 0.750 M NaOH solution. How many ml of NaOH are required to
reach the third equivalence point with a starting volume of 25.0 mL H:CGHSO,
according to the following balanced chemical equation:
H:C&H:O, + 3 NaOH – NasCeHsO, +
3 H:0
25.0
mL H:C&HSO7
moLNaOH
molH.eHsO,
20.0
mL NAOH
0.200
mo
molHeH50,
molAaOH
LH:CCHSO7
1
1
0.750
0.200 mol H:CH:O,
25.0 mL H:CeH:O,
3 mol NaOH
1 mol H.CcHsO,
20.0 mL NaOH
LH:CcHsO7
1 mol H:CeHsO,
1 mol H:C&HsO,
0.750 mol NaOH
Incorrect, 0 attempts remaining
The correct answer is 20.0 mL NaOH.
Your ratio between mL H:CsH:O; and mol HsCsHsO, in your first conversion factor is
incorrect. Check the problem statement and then try to determine the correct values
for the factor.
Your mole ratio between H:C&HSO; and NaOH in your third conversion factor is
incorrect. Reconsider the number of mol of HsC&HsO, per mol of NaOH. Checking the
coefficients in the given balanced reaction may help you determine the correct mole
ratio
3:09 PM
P Type here to search
中)
4/28/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax