plem Set #5 - The following questions refer to the chart below which shows the II) Noncovalent Forces boiling points of specific compounds containing only H-E bonds, where E stands for elements in various periods and groups. 150 a) Explain the steady increase in boiling points in going from Period 3 to Period 5 elements. H2O 100 50 HFO H2TE SBH3 NH3 H2Se HI H2S AsH HCI –50 b) Explain the sharp decrease in boiling points in going from Period 2 to Period 3 elements. HBr -100 PH Group 17 Group 16 Group 15 -150 2 4. Period c) Do you expect melting points to necessarily follow the same trends? Why or why not? Boiling point (°C)
plem Set #5 - The following questions refer to the chart below which shows the II) Noncovalent Forces boiling points of specific compounds containing only H-E bonds, where E stands for elements in various periods and groups. 150 a) Explain the steady increase in boiling points in going from Period 3 to Period 5 elements. H2O 100 50 HFO H2TE SBH3 NH3 H2Se HI H2S AsH HCI –50 b) Explain the sharp decrease in boiling points in going from Period 2 to Period 3 elements. HBr -100 PH Group 17 Group 16 Group 15 -150 2 4. Period c) Do you expect melting points to necessarily follow the same trends? Why or why not? Boiling point (°C)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 38QRT
Related questions
Question
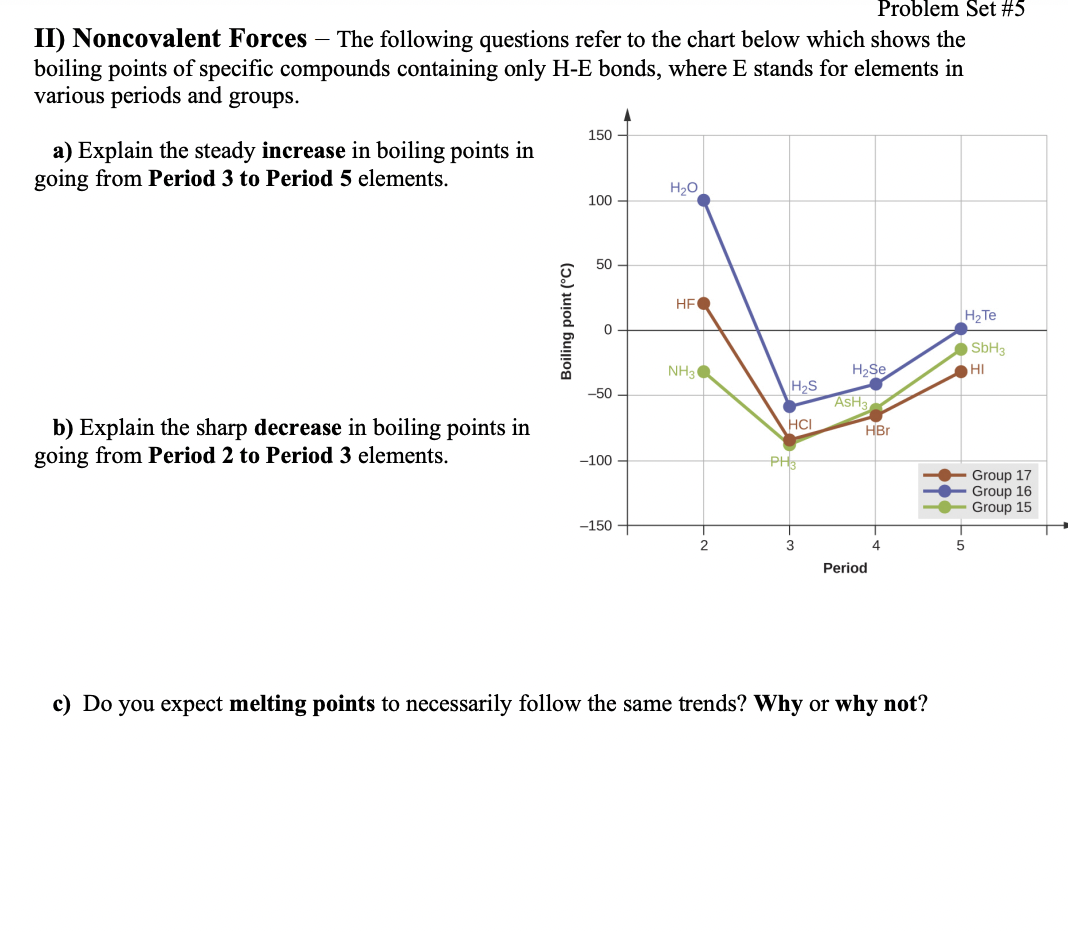
Transcribed Image Text:Problem Set #5
II) Noncovalent Forces
boiling points of specific compounds containing only H-E bonds, where E stands for elements in
various periods and groups.
- The following questions refer to the chart below which shows the
150
a) Explain the steady increase in boiling points in
going from Period 3 to Period 5 elements.
H20
100
50
НЕО
SBH3
NH3
H2Se
HI
H2S
ASH3
-50
b) Explain the sharp decrease in boiling points in
going from Period 2 to Period 3 elements.
HCI
HBr
-100
Group 17
Group 16
Group 15
-150
3
4
5
Period
c) Do you expect melting points to necessarily follow the same trends? Why or why not?
Boiling point (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning