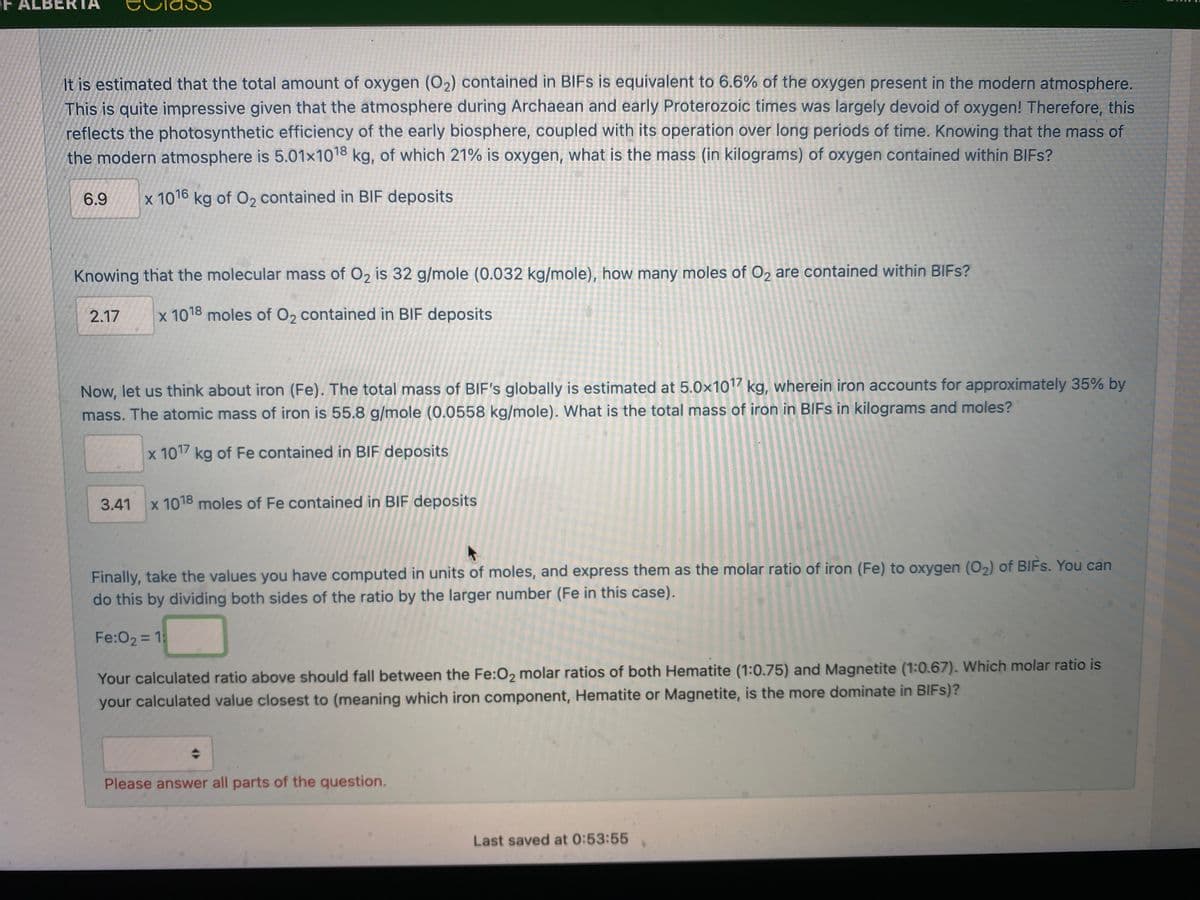
It is estimated that the total amount of oxygen (O2) contained in BIFS is equivalent to 6.6% of the oxygen present in the modern atmosphere. This is quite impressive given that the atmosphere during Archaean and early Proterozoic times was largely devoid of oxygen! Therefore, this reflects the photosynthetic efficiency of the early biosphere, coupled with its operation over long periods of time. Knowing that the mass of the modern atmosphere is 5.01x1018 kg, of which 21% is oxygen, what is the mass (in kilograms) of oxygen contained within BIFS? 6.9 x 1016 kg of O2 contained in BIF deposits Knowing that the molecular mass of O2 is 32 g/mole (0.032 kg/mole), how many moles of O2 are contained within BIFS? 2.17 x 1018 moles of O2 contained in BIF deposits Now, let us think about iron (Fe). The total mass of BIF's globally is estimated at 5.0×1017 kg, wherein iron accounts for approximately 35% by mass. The atomic mass of iron is 55.8 g/mole (0.0558 kg/mole). What is the total mass of iron in BIFS in kilograms and moles? x 1017 kg of Fe contained in BIF deposits 3.41 x 1018 moles of Fe contained in BIF deposits Finally, take the values you have computed in units of moles, and express them as the molar ratio of iron (Fe) to oxygen (O2) of BIFS. You can do this by dividing both sides of the ratio by the larger number (Fe in this case). Fe:O2 = 1 Your calculated ratio above should fall between the Fe:02 molar ratios of both Hematite (1:0.75) and Magnetite (1:0.67). Which molar ratio is your calculated value closest to (meaning which iron component, Hematite or Magnetite, is the more dominate in BIIFS)? Please answer all parts of the question.
It is estimated that the total amount of oxygen (O2) contained in BIFS is equivalent to 6.6% of the oxygen present in the modern atmosphere. This is quite impressive given that the atmosphere during Archaean and early Proterozoic times was largely devoid of oxygen! Therefore, this reflects the photosynthetic efficiency of the early biosphere, coupled with its operation over long periods of time. Knowing that the mass of the modern atmosphere is 5.01x1018 kg, of which 21% is oxygen, what is the mass (in kilograms) of oxygen contained within BIFS? 6.9 x 1016 kg of O2 contained in BIF deposits Knowing that the molecular mass of O2 is 32 g/mole (0.032 kg/mole), how many moles of O2 are contained within BIFS? 2.17 x 1018 moles of O2 contained in BIF deposits Now, let us think about iron (Fe). The total mass of BIF's globally is estimated at 5.0×1017 kg, wherein iron accounts for approximately 35% by mass. The atomic mass of iron is 55.8 g/mole (0.0558 kg/mole). What is the total mass of iron in BIFS in kilograms and moles? x 1017 kg of Fe contained in BIF deposits 3.41 x 1018 moles of Fe contained in BIF deposits Finally, take the values you have computed in units of moles, and express them as the molar ratio of iron (Fe) to oxygen (O2) of BIFS. You can do this by dividing both sides of the ratio by the larger number (Fe in this case). Fe:O2 = 1 Your calculated ratio above should fall between the Fe:02 molar ratios of both Hematite (1:0.75) and Magnetite (1:0.67). Which molar ratio is your calculated value closest to (meaning which iron component, Hematite or Magnetite, is the more dominate in BIIFS)? Please answer all parts of the question.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 85E: Propane, C3H8, is a hydrocarbon that is commonly used as a fuel. (a) Write a balanced equation for...
Related questions
Question
100%
Transcribed Image Text:FALBERTA
It is estimated that the total amount of oxygen (O2) contained in BIFS is equivalent to 6.6% of the oxygen present in the modern atmosphere.
This is quite impressive given that the atmosphere during Archaean and early Proterozoic times was largely devoid of oxygen! Therefore, this
reflects the photosynthetic efficiency of the early biosphere, coupled with its operation over long periods of time. Knowing that the mass of
the modern atmosphere is 5.01×1018 kg, of which 21% is oxygen, what is the mass (in kilograms) of oxygen contained within BIFS?
6.9
x 1016 kg of O2 contained in BIF deposits
Knowing that the molecular mass of O2 is 32 g/mole (0.032 kg/mole), how many moles of O2 are contained within BIFS?
2.17
x 1018 moles of O2 contained in BIF deposits
Now, let us think about iron (Fe). The total mass of BIF's globally is estimated at 5.0x1017 kg, wherein iron accounts for approximately 35% by
mass. The atomic mass of iron is 55.8 g/mole (0.0558 kg/mole). What is the total mass of iron in BIFS in kilograms and moles?
x 1017 kg of Fe contained in BIF deposits
3.41
x 1018 moles of Fe contained in BIF deposits
Finally, take the values you have computed in units of moles, and express them as the molar ratio of iron (Fe) to oxygen (O2) of BIFS. You can
do this by dividing both sides of the ratio by the larger number (Fe in this case).
Fe:O2 = 1:
Your calculated ratio above should fall between the Fe:02 molar ratios of both Hematite (1:0.75) and Magnetite (1:0.67). Which molar ratio is
your calculated value closest to (meaning which iron component, Hematite or Magnetite, is the more dominate in BIFS)?
Please answer all parts of the question.
Last saved at 0:53:55
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER