Point 1: Point 2: -1 material balance (H₂O-dry air is already balanced on the chart) -2 absolute humidities from psychrometric chart (for inlet and outlet air) -2 enthalpies from psychrometric chart (for inlet and outlet air) -1 enthalpy of condensate (from known heat capacity of liquid water) -1 energy balance =0 degrees of freedom Balance on H₂O: Fraction H₂0 Condensed: 80°F 80% RH 1.0 51°F) Saturated Figure 1.4-2 h = 0.018 lb H₂O/lb DA H₁ 38.8 Btu/lb, DA DA 842 0.018 lb H₂O -0.018b H₂O lb DA 1.0lb DA 0.0079 lb H₂O Ib,,, ᎠᎪ forate on the us psychso. 0.018 -0.0079 my=0.010 lb H₂O condensed 0.010 lb condensed 0.018 lb, fed k₂ = 0.0079 lb H₂O/lb DAN H₂= 20.9 Btu/lb DA -0.0079 lb H₂O Chart "Docate on us psychro. chart =0.555
Point 1: Point 2: -1 material balance (H₂O-dry air is already balanced on the chart) -2 absolute humidities from psychrometric chart (for inlet and outlet air) -2 enthalpies from psychrometric chart (for inlet and outlet air) -1 enthalpy of condensate (from known heat capacity of liquid water) -1 energy balance =0 degrees of freedom Balance on H₂O: Fraction H₂0 Condensed: 80°F 80% RH 1.0 51°F) Saturated Figure 1.4-2 h = 0.018 lb H₂O/lb DA H₁ 38.8 Btu/lb, DA DA 842 0.018 lb H₂O -0.018b H₂O lb DA 1.0lb DA 0.0079 lb H₂O Ib,,, ᎠᎪ forate on the us psychso. 0.018 -0.0079 my=0.010 lb H₂O condensed 0.010 lb condensed 0.018 lb, fed k₂ = 0.0079 lb H₂O/lb DAN H₂= 20.9 Btu/lb DA -0.0079 lb H₂O Chart "Docate on us psychro. chart =0.555
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Locate the two points and humid volume on the US psychrometric chart.
Transcribed Image Text:||!
PDF Elementary Principles of Chemica X PDF *Elementary Principles of Chemic X +
76°F
Mostly sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
(T)
Read aloud
Test Yourself
(Answers, p. 659)
Q Search
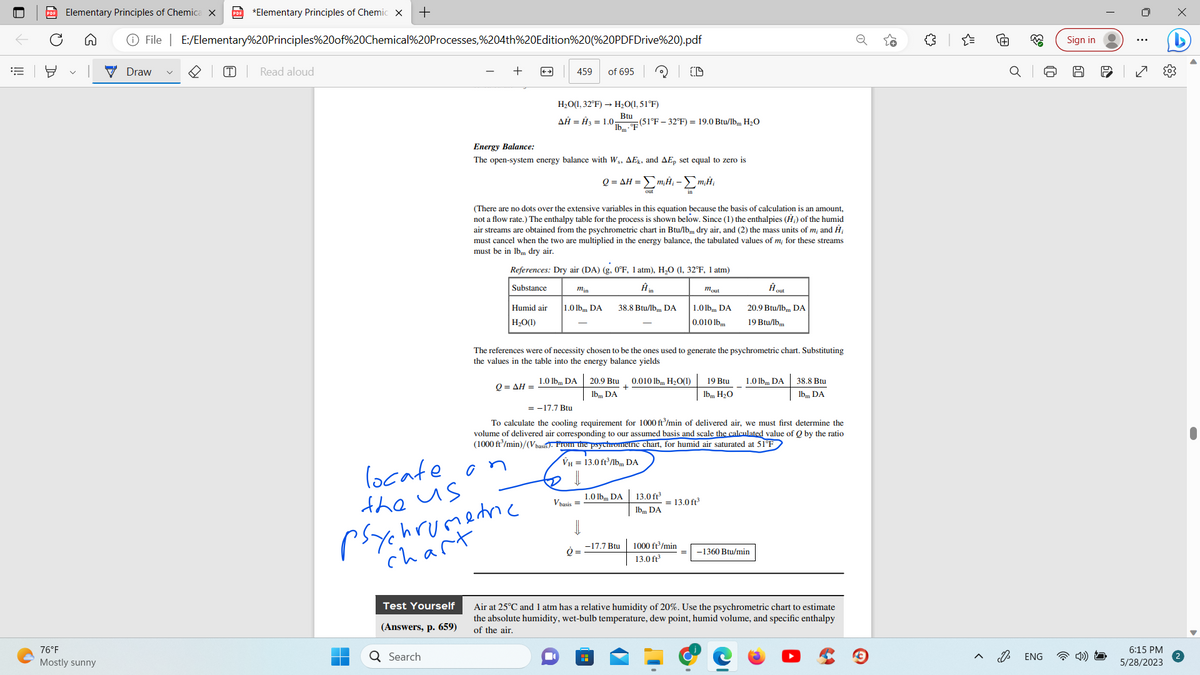
Energy Balance:
The open-system energy balance with Ws, AEk, and AEp set equal to zero is
m₂Ĥ₁
Q=AH =
= Σm.#₁ - Σm
out
(There are no dots over the extensive variables in this equation because the basis of calculation is an amount,
not a flow rate.) The enthalpy table for the process is shown below. Since (1) the enthalpies (H₂) of the humid
air streams are obtained from the psychrometric chart in Btu/lb dry air, and (2) the mass units of m; and Ĥ₂
must cancel when the two are multiplied in the energy balance, the tabulated values of m; for these streams
must be in lbm dry air.
locate a
the us
psychrumetric
chart
459 of 695
Humid air
H₂O(1)
Q=AH =
on
H₂O(1, 32°F)→ H₂O(1,51°F)
Btu
AHH₂= 1.0; (51°F 32°F) = 19.0 Btu/lbm H₂O
lb °F
References: Dry air (DA) (g. 0°F, 1 atm), H₂O (1, 32°F, 1 atm)
Substance
min
1.0 lbm DA
The references were of necessity chosen to be the ones used to generate the psychrometric chart. Substituting
the values in the table into the energy balance yields
1.0 lbm DA
■
Vbasis =
38.8 Btu/lbm DA
Q=
20.9 Btu 0.010 lbm H₂O(1)
+
lbm DA
= -17.7 Btu
To calculate the cooling requirement for 1000 ft³/min of delivered air, we must first determine the
volume of delivered air corresponding to our assumed basis and scale the calculated value of Q by the ratio
(1000 ft³/min)/(Vbasis). From the psychrometric chart, for humid air saturated at 51°F
VH = 13.0 ft³/lbm DA
1.0 lbm DA
-17.7 Btu
mout
1.0lbm DA
0.010 lbm
13.0 ft³
= 13.0 ft³
lbm DA
1000 ft³/min
13.0 ft³
Ĥ out
20.9 Btu/lbm DA
19 Btu/lbm
19 Btu
lbm H₂O
1.0 lbm DA
38.8 Btu
lbm DA
-1360 Btu/min
Air at 25°C and 1 atm has a relative humidity of 20%. Use the psychrometric chart to estimate
the absolute humidity, wet-bulb temperature, dew point, humid volume, and specific enthalpy
of the air.
o
{"
@
683
ENG
Sign in
00
:
6:15 PM
5/28/2023
Transcribed Image Text:||!
PDF Elementary Principles of Chemica X PDF *Elementary Principles of Chemic X +
76°F
Mostly sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
(T)
Read aloud
Example 8.4-6
Equipment Encyclopedia
heat exchanger
www.wiley.com/college/felder
Solution
Q Search
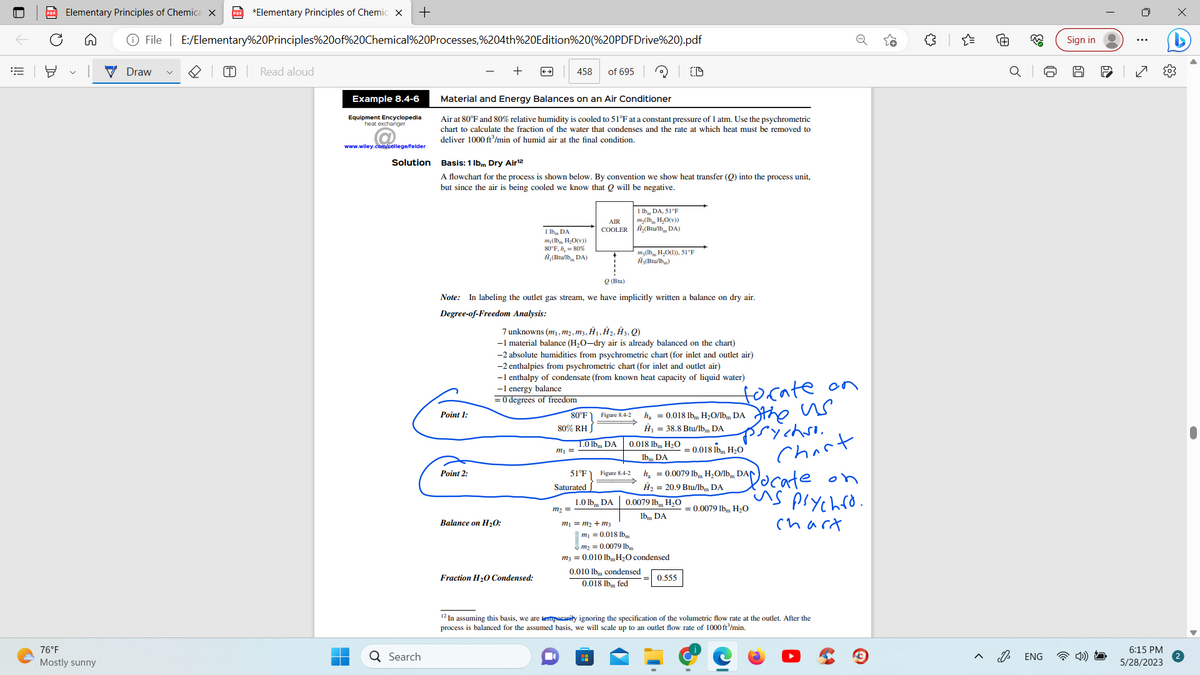
Material and Energy Balances on an Air Conditioner
Air at 80°F and 80% relative humidity is cooled to 51°F at a constant pressure of 1 atm. Use the psychrometric
chart to calculate the fraction of the water that condenses and the rate at which heat must be removed to
deliver 1000 ft³/min of humid air at the final condition.
Basis: 1 lbm Dry Air¹2
A flowchart for the process is shown below. By convention we show heat transfer (Q) into the process unit,
but since the air is being cooled we know that Q will be negative.
Point 1:
Point 2:
458 of 695 3
1 lbm DA
mi(lbm H₂O(v))
80°F, h, = 80%
Ĥ₁ (Btu/lb DA)
Q (Btu)
Note: In labeling the outlet gas stream, we have implicitly written a balance on dry air.
Degree-of-Freedom Analysis:
Balance on H₂O0:
Fraction H₂O Condensed:
7 unknowns (m₁, m2, m3, H1, H2, H3, Q)
-1 material balance (H₂O-dry air is already balanced on the chart)
-2 absolute humidities from psychrometric chart (for inlet and outlet air)
-2 enthalpies from psychrometric chart (for inlet and outlet air)
-1 enthalpy of condensate (from known heat capacity of liquid water)
-1 energy balance
= 0 degrees of freedom
80% RH
m₁ =
m₂ =
■
AIR
COOLER
80°F) Figure 8.4-2
51°F
Saturated
1.0 lbm DA
1 lb DA, 51°F
m₂(lb H₂O(v))
H₂(Btu/lb DA)
1.0 lbm DA
m3(lb H₂O(1)), 51°F
Å3(Btu/lb.)
Figure 8.4-2
m₁ = m₂ + m3
H
0.018 lbm H₂O
lbm DA
h₂0.018 lbm H₂O/1bm DA
Ĥ₁ = 38.8 Btu/lbm DA
0.0079 lbm H₂O
lbm DA
m₁ = 0.018 lbm
m₂ = 0.00791
m3 = 0.010 lbm H₂O condensed
0.010 lbm condensed
0.018 lbm fed
forate on
the us
psychso.
h₂ = 0.0079 lbm H₂O/lb DAN
Ĥ₂ = 20.9 Btu/lbm DA
0.555
= 0.018 lbm H₂O
chart
DADocate on
is psychro.
chart
12 In assuming this basis, we are temporarily ignoring the specification of the volumetric flow rate at the outlet. After the
process balanced for the assumed basis, we will scale up to an outlet flow rate of 1000 ft³/min.
o
= 0.0079 lbm H₂O
{"
@
683
ENG
Sign in
00
:
6:15 PM
5/28/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The