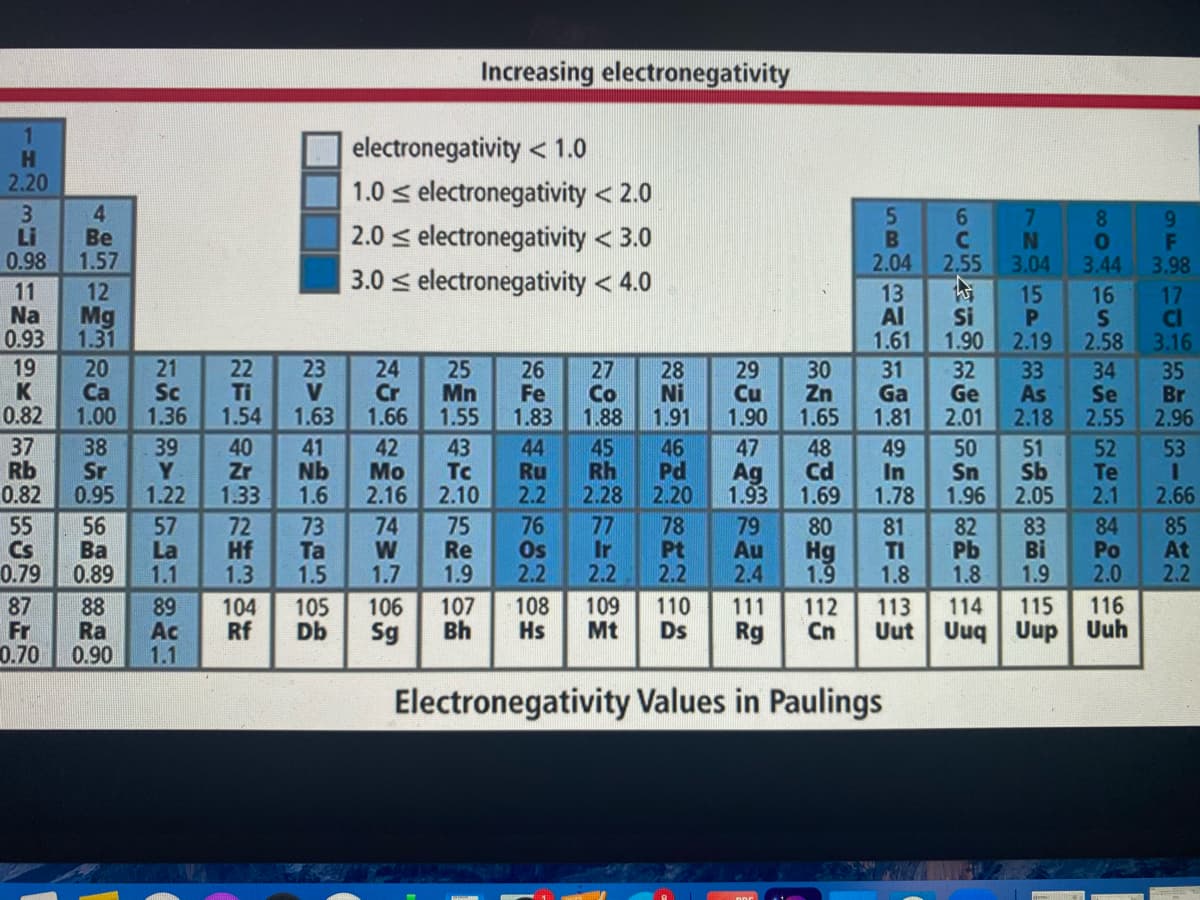
POS Using the electronegativity values (table below), which of the following bonds is the most polar covalent without being considered ionic? O H-S O Cu-S O Ca-CI O H-O
POS Using the electronegativity values (table below), which of the following bonds is the most polar covalent without being considered ionic? O H-S O Cu-S O Ca-CI O H-O
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 85QRT
Related questions
Question
Can you help me solve this
Transcribed Image Text:Increasing electronegativity
electronegativity < 1.0
HA
2.20
1.0 s electronegativity < 2.0
4.
Be
1.57
Li
0.98
2.0 s electronegativity < 3.0
N
3.04
2.04
2.55
3.44
3.98
3.0 s electronegativity < 4.0
11
Na
0.93
19
K
0.82
12
Mg
13
Al
1.61
15
16
Si
1.90
17
cl
3.16
1.31
2.19
32
Ge
2.01
2.58
20
Ca
1.00
21
Sc
1.36
22
Ti
1.54
23
V
1.63
24
Cr
1.66
25
Mn
1.55
26
Fe
1.83
27
Co
1.88
28
Ni
1.91
29
Cu
1.90
30
Zn
1.65
31
Ga
1.81
33
As
2.18
34
Se
2.55
35
Br
2.96
37
Rb
0.82
38
Sr
0.95
39
40
Zr
1.33
41
Nb
1.6
42
Мо
2.16
43
Tc
2.10
44
Ru
2.2
45
Rh
2.28
46
Pd
2.20
47
Ag
1.93
48
Cd
1.69
49
In
1.78
50
Sn
51
Sb
2.05
52
Te
2.1
53
1.22
1.96
2.66
55
Cs
0.79
56
Ba
0.89
57
La
1.1
72
Hf
1.3
74
73
Tа
1.5
75
Re
1.9
76
Os
2.2
77
Ir
2.2
78
Pt
2.2
79
Au
2.4
80
Hg
1.9
81
TI
1.8
82
Pb
1.8
83
Bi
1.9
84
Po
2.0
85
At
2.2
1.7
116
87
Fr
0.70
88
Ra
0.90
89
Ac
1.1
106
107
Bh
108
Hs
109
Mt
110
Ds
114
115
104
Rf
105
Db
111
Sg
112
Cn
113
Uut
Rg
Uuq
Uup Uuh
Electronegativity Values in Paulings
408
49

Transcribed Image Text:POS
Using the electronegativity values (table below), which of the following bonds is the most polar covalent without being considered ionic?
O H-S
O Cu-S
O Ca-CI
O H-O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning