Potassium chlorate, potassium chloride, and water is produced by passing chlorine gas into a hot solution of potassium hydroxide. Calculate the volume in L of chlorine gas needed to produce 724.25 mL of 4.25 M potassium chlorate. The density of chlorine gas is 3.17 g/L.
Potassium chlorate, potassium chloride, and water is produced by passing chlorine gas into a hot solution of potassium hydroxide. Calculate the volume in L of chlorine gas needed to produce 724.25 mL of 4.25 M potassium chlorate. The density of chlorine gas is 3.17 g/L.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 33CR: If an electric current is passed through molten sodium chloride, elemental chlorine gas is generated...
Related questions
Question
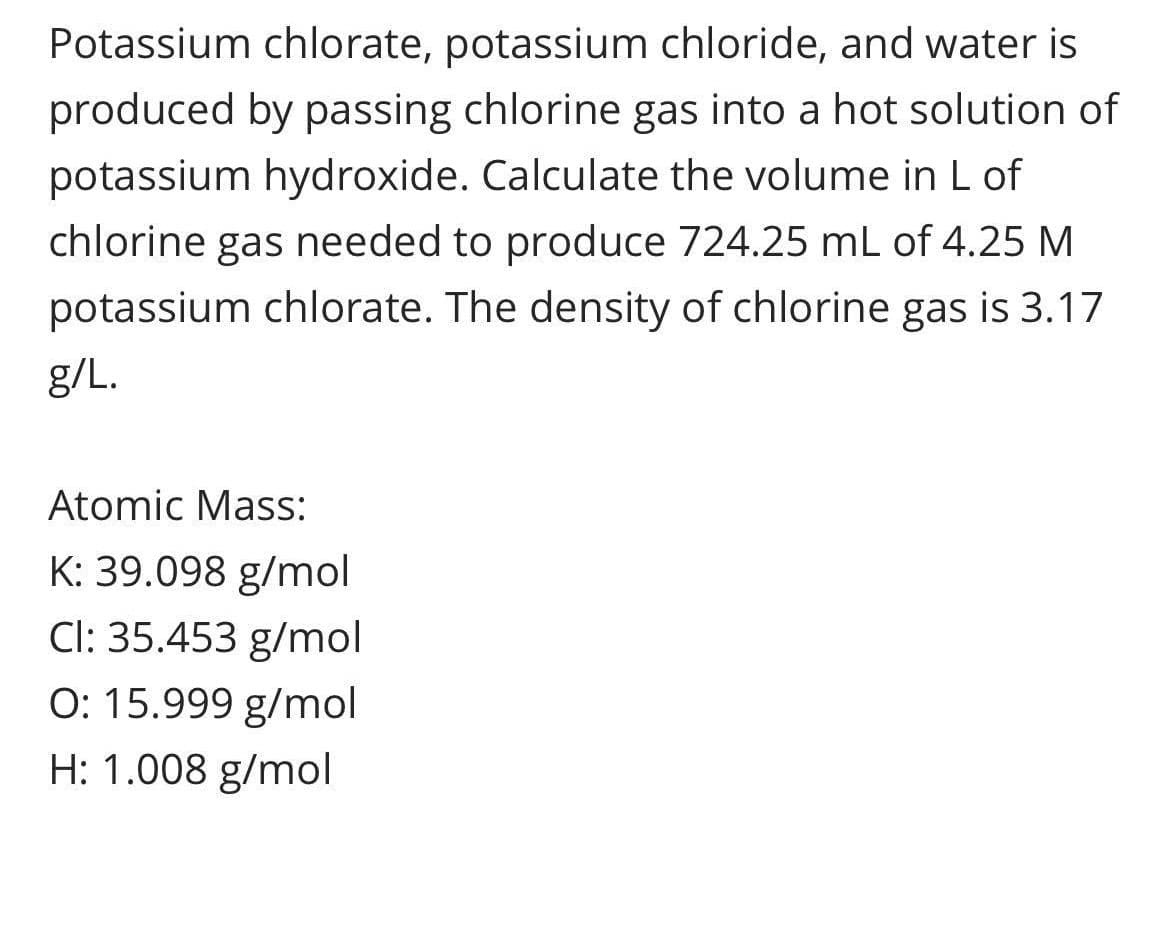
Transcribed Image Text:Potassium chlorate, potassium chloride, and water is
produced by passing chlorine gas into a hot solution of
potassium hydroxide. Calculate the volume in L of
chlorine gas needed to produce 724.25 mL of 4.25 M
potassium chlorate. The density of chlorine gas is 3.17
g/L.
Atomic Mass:
K: 39.098 g/mol
Cl: 35.453 g/mol
O: 15.999 g/mol
H: 1.008 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning