Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 1QAP
Related questions
Question
100%
Transcribed Image Text:C A Glass Container Was Initially X
b Search results for 'A glass con xG Aglass container was initially x +
101
* M
i app.101edu.co
t1 Reading List
G M Gmal o YouTube Q Maos a AMAZON A Translate oflights 8 UsCis h BATERBLY a CHEGG KATAPULK CUBA SUPERMARKET23 4 Essay Writing Ser
a AMAZON Translate à onights 3 uscIs b BATERBLY C CHEGG >K KATAPULK CUBA SUPERMARKET23 + Essay Writing Ser. G calculator - Googl
Submit
Question 18 of 25
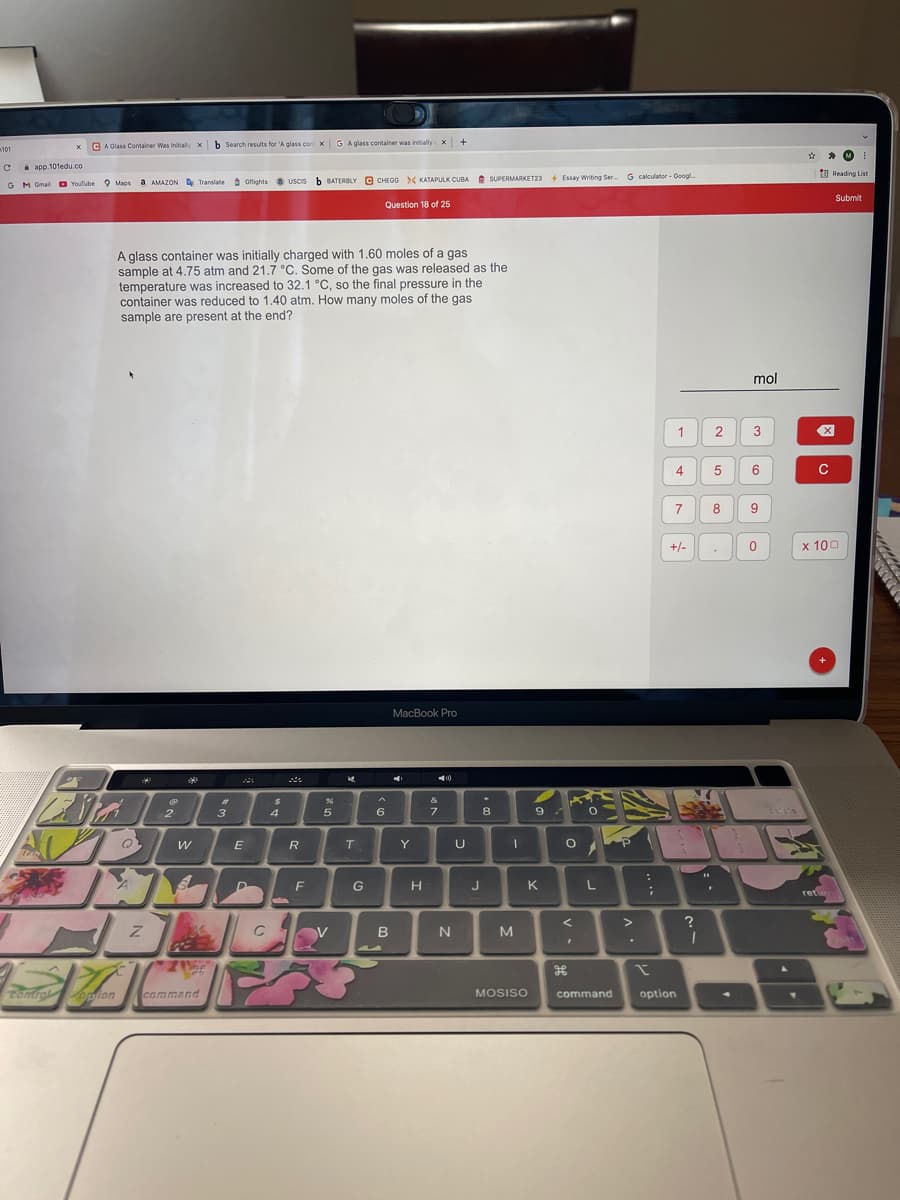
A glass container was initially charged with 1.60 moles of a gas
sample at 4.75 atm and 21.7 °C. Some of the gas was released as the
temperature was increased to 32.1 °C, so the final pressure in the
container was reduced to 1.40 atm. How many moles of the gas
sample are present at the end?
mol
1
2
3
4
5
6
C
7
8
+/-
х 100
MacBook Pro
&
%23
3
2
7
8.
9
E
R
T.
Y
G
H
J
K
L
ret
M
Tomroloplon
command
MOSISO
command
option
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
