Use the balanced equation to answer the question follows. 2C2H6(g)+5O2(g) -----> 4CO(g)+6H2O(g) to calculate how many moles of O2 are needed to react completely with 1.6 moles of C2H6?
Use the balanced equation to answer the question follows. 2C2H6(g)+5O2(g) -----> 4CO(g)+6H2O(g) to calculate how many moles of O2 are needed to react completely with 1.6 moles of C2H6?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter3: Equation, The Mole, And Chemical Formulas
Section: Chapter Questions
Problem 3.123QE: Aluminum metal reacts with sulfuric acid, H2SO4, to yield aluminum sulfate and hydrogen gas....
Related questions
Question
Use the balanced equation to answer the question follows. 2C2H6(g)+5O2(g) -----> 4CO(g)+6H2O(g) to calculate how many moles of O2 are needed to react completely with 1.6 moles of C2H6?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Would you be able to show me how to set it up like this? The second image is an example.

Transcribed Image Text:Conversion factors.
2.) 2 C₂ H₂(g) +50₂(g) → 4 (0(g) +6₂ H₂O(g)
quantity
1.6 moles of C₂tl₂
Units desired
moles of 0₂
Conversion factors
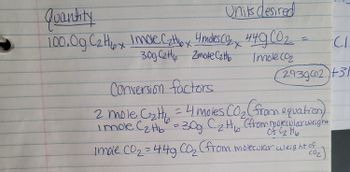
Transcribed Image Text:Quantity
Units desired
100.0g C₂H₂px Imole C₂H6x 4 moles Co₂x 449 CO₂
30g Czty Zmote Catto
Imole (0₂
Conversion factors
2 mole. C₂tt₂ = 4 moles CO₂ (from equation)
i mole C₂H₂ = 30g C₂H₁ (from molecular weight
Imole CO₂ = 44g CO₂₂ (from molecular weight of
CO₂)
CI
293902 +31
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
