Predicting relative forward and reverse rates of reaction in a dynemic equilibrium Hydrogen and chlorine react to form hydrogen chloride, like this: H₂(0)+Cl₂(g)-2 HCl(g) Imagine 113. mmol of HC1 are added to an empty flask, and then answer the fa What is the rate of the reverse reaction before any HC has been added to the flask? What is the rate of the reverse reaction just after the HCI has been added to the flask? What is the rate of the reverse reaction at equilibrium? How much HCI is in the flask at equilibrium? Zero. Greater than zere, but less than the rate of Greater than zers, and equal to the rate of Greeter than zera, and greater than the ram - Zero. Greater than zers, but less than the rate of Greater than zers, and equal to the rate of Greater than zers, and greater than the rac Zero. Greater than zers, but less than the rate Greater dan zers, and equal to the rate of Greater than zers, and greater than the rat None. Some, but less than 113. mmol. 113. mmol
Predicting relative forward and reverse rates of reaction in a dynemic equilibrium Hydrogen and chlorine react to form hydrogen chloride, like this: H₂(0)+Cl₂(g)-2 HCl(g) Imagine 113. mmol of HC1 are added to an empty flask, and then answer the fa What is the rate of the reverse reaction before any HC has been added to the flask? What is the rate of the reverse reaction just after the HCI has been added to the flask? What is the rate of the reverse reaction at equilibrium? How much HCI is in the flask at equilibrium? Zero. Greater than zere, but less than the rate of Greater than zers, and equal to the rate of Greeter than zera, and greater than the ram - Zero. Greater than zers, but less than the rate of Greater than zers, and equal to the rate of Greater than zers, and greater than the rac Zero. Greater than zers, but less than the rate Greater dan zers, and equal to the rate of Greater than zers, and greater than the rat None. Some, but less than 113. mmol. 113. mmol
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter18: Chemical Kinetics
Section: Chapter Questions
Problem 66AP
Related questions
Question
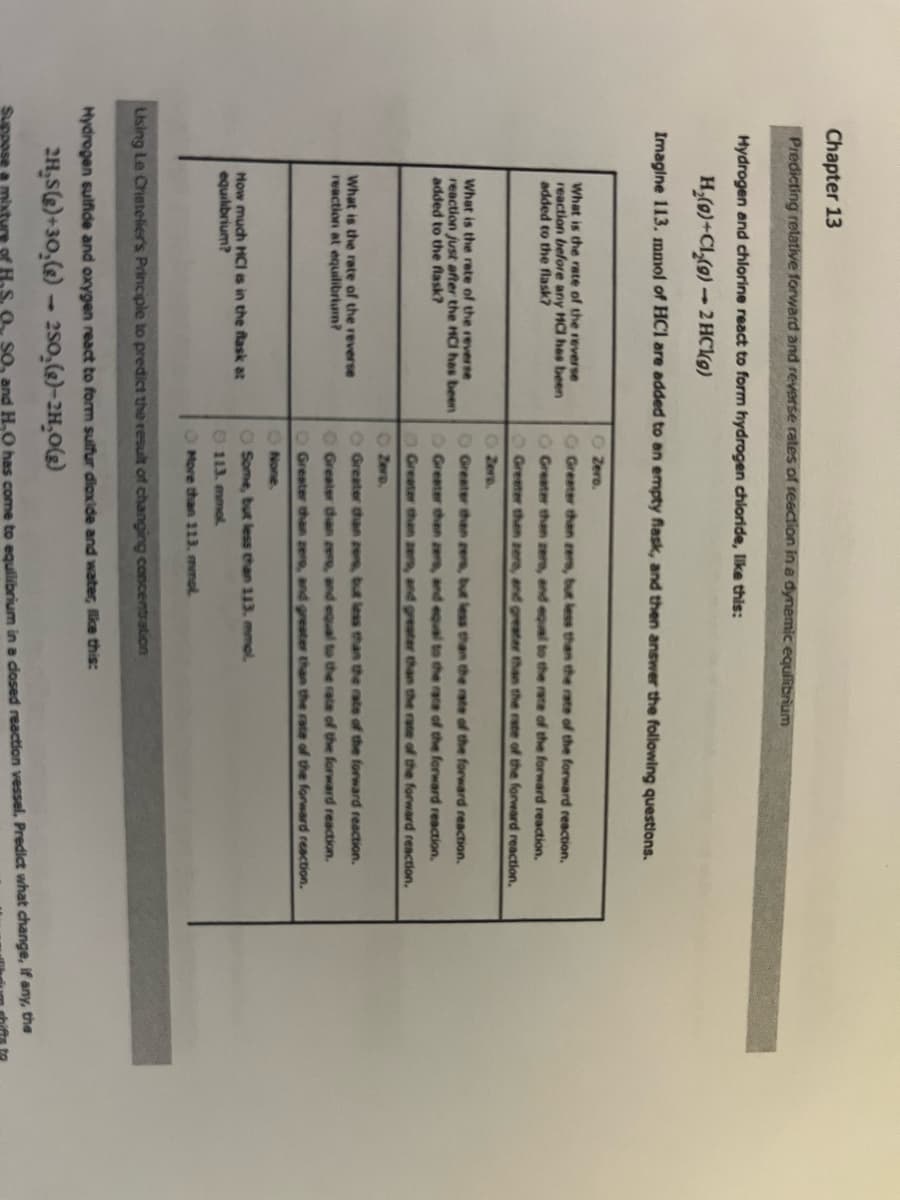
Transcribed Image Text:Chapter 13
Predicting relative forward and reverse rates of reaction in a dynamic equilibrium
Hydrogen and chlorine react to form hydrogen chloride, like this:
H₂(g)+Cl₂(g)-2 HCl(g)
Imagine 113. mmol of HC1 are added to an empty flask, and then answer the following questions.
What is the rate of the reverse
reaction before any HC has been
added to the flask?
What is the rate of the reverse
reaction just after the HCI has been
added to the flask?
What is the rate of the reverse
reaction at equilibrium?
How much HCI is in the flask at
equilibrium?
Zero.
Greater than zere, but less than the rate of the forward reaction.
Greater than zero, and equal to the rate of the forward reaction.
Greater than zera, and greater than the rate of the forward reaction.
Zers.
Greater than zers, but less than the rate of the forward reaction.
Greater than zers, and equal to the race of the forward reaction.
Greater than zers, and greater than the rate of the forward reaction.
Zero.
O Greater than zero, but less than the rate of the forward reaction.
Greater than cer, and equal to the rate of the forward reaction.
O Greater than zero, and greater than the rate of the forward reaction.
None.
O Some, but less than 113. mmol.
113. mmol
More than 113. mmol
Using Le Chatelier's Principle to predict the result of changing concentration
Hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this:
2H₂S(g)+30,(g)
250,(g)-2H₂O(g)
SO, and H₂O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning