Problem 1. An ideal gas with molar mass M (mass in kg per one mole) is kept at pressure p and temperature T. If the mean free path of its molecules is Ao, find the density of the gas, number of its molecules in m (per cubic meter) and effective volume of a molecule of this gas.
Problem 1. An ideal gas with molar mass M (mass in kg per one mole) is kept at pressure p and temperature T. If the mean free path of its molecules is Ao, find the density of the gas, number of its molecules in m (per cubic meter) and effective volume of a molecule of this gas.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section8.8: The Behavior Of Real (non-ideal) Gases
Problem 8.18E
Related questions
Question
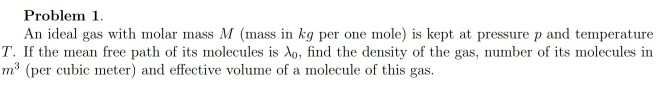
Transcribed Image Text:Problem 1.
An ideal gas with molar mass M (mass in kg per one mole) is kept at pressure p and temperature
T. If the mean free path of its molecules is Ao, find the density of the gas, number of its molecules in
m (per cubic meter) and effective volume of a molecule of this gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning