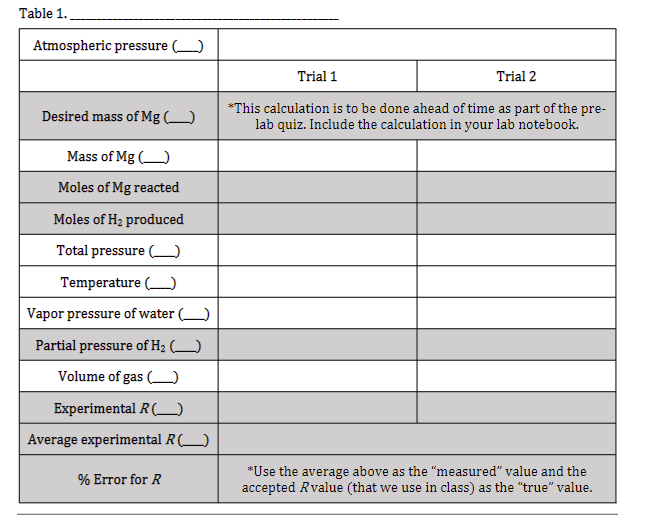
desired Mg mass =0.0340g "Atmospheric pressure (mmHg): 733.59 Trial 1 and Trial 2 values separated by commas: Mass of Mg (g): 0.038, 0.031 Temperature (°C): 20.2 (both trials) Vapor pressure of water (mmHg): 17.86 (both trials) Volume of gas (mL): 42.95, 35.80 need calculations for table.
desired Mg mass =0.0340g "Atmospheric pressure (mmHg): 733.59 Trial 1 and Trial 2 values separated by commas: Mass of Mg (g): 0.038, 0.031 Temperature (°C): 20.2 (both trials) Vapor pressure of water (mmHg): 17.86 (both trials) Volume of gas (mL): 42.95, 35.80 need calculations for table.
Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.103E
Related questions
Question
desired Mg mass =0.0340g
"Atmospheric pressure (mmHg): 733.59
Trial 1 and Trial 2 values separated by commas:
Mass of Mg (g): 0.038, 0.031
Temperature (°C): 20.2 (both trials)
Vapor pressure of water (mmHg): 17.86 (both trials)
Volume of gas (mL): 42.95, 35.80
need calculations for table.
Transcribed Image Text:Table 1.
Atmospheric pressure (______)
Desired mass of Mg (_____)
Mass of Mg()
Moles of Mg reacted
Moles of H₂ produced
Total pressure (
Temperature (
Vapor pressure of water (_____)
Partial pressure of H₂ (____)
Volume of gas
Experimental R (_____)
Average experimental R (______)
% Error for R
Trial 1
Trial 2
*This calculation is to be done ahead of time as part of the pre-
lab quiz. Include the calculation in your lab notebook.
*Use the average above as the "measured" value and the
accepted Rvalue (that we use in class) as the "true" value.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
can you show the expermiental r calculations in detail
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning