Problem 19.28 11 of 16 Constants I Periodic Ta A gas is enclosed in a cylinder fitted with a light frictionless piston and maintained at atmospheric pressure. When 9950 kcal of heat is added to the gas, the volume is observed to Part A increase slowly from 13.7 m3 to 18.8 m3 Calculate the work done by the gas. Express your answer using two significant figures. nν ΑΣφ Submit Request Answer Part B Calculate the change in internal energy of the gas. να ΑΣφ AEint = Submit Request Answer
Problem 19.28 11 of 16 Constants I Periodic Ta A gas is enclosed in a cylinder fitted with a light frictionless piston and maintained at atmospheric pressure. When 9950 kcal of heat is added to the gas, the volume is observed to Part A increase slowly from 13.7 m3 to 18.8 m3 Calculate the work done by the gas. Express your answer using two significant figures. nν ΑΣφ Submit Request Answer Part B Calculate the change in internal energy of the gas. να ΑΣφ AEint = Submit Request Answer
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 9CQ: Calculate the length of a 1-meter rod of a material with thermal expansion coefficient a when the...
Related questions
Question
Please help me with all the parts of this question, please also double check your answers as previous tutors got it wrong.
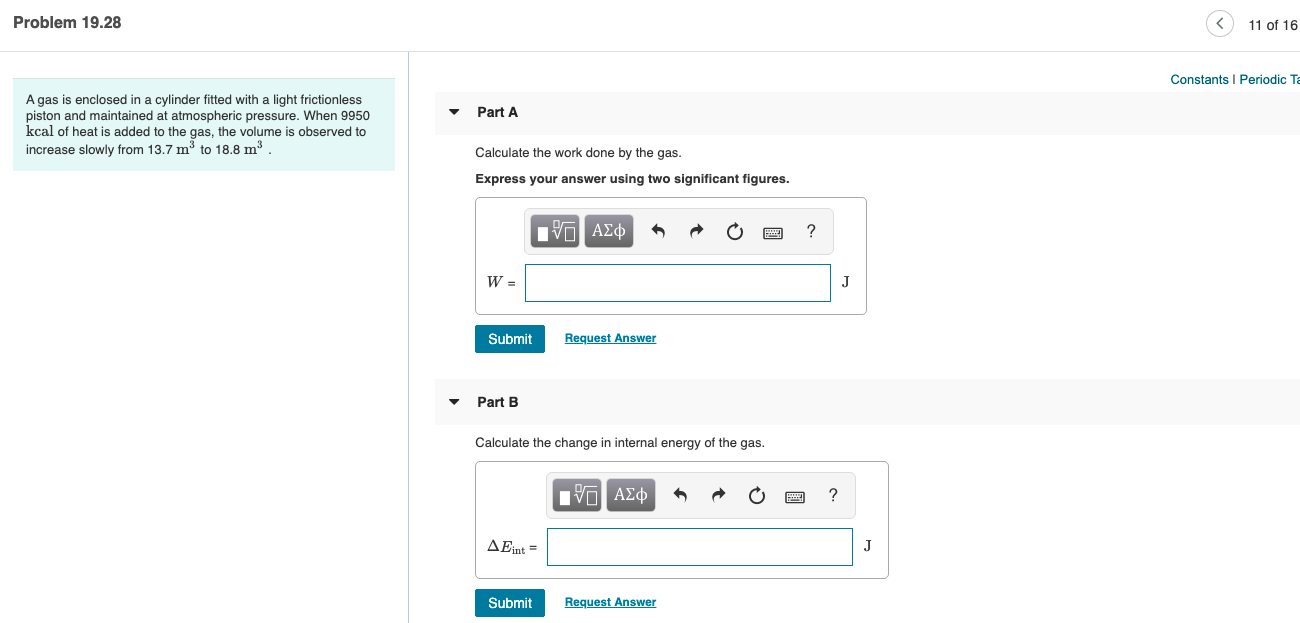
Transcribed Image Text:Problem 19.28
11 of 16
Constants I Periodic Ta
A gas is enclosed in a cylinder fitted with a light frictionless
piston and maintained at atmospheric pressure. When 9950
kcal of heat is added to the gas, the volume is observed to
Part A
increase slowly from 13.7 m3 to 18.8 m3
Calculate the work done by the gas.
Express your answer using two significant figures.
nν ΑΣφ
Submit
Request Answer
Part B
Calculate the change in internal energy of the gas.
να ΑΣφ
AEint =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning