Problem 22 22 of 27 > Scuba divers who suffer from decompression sickness are treated in hyperbaric chambers using heliox (21 % Oxygen, 79% helium) at pressures up to 150 psi Review I Constants I Periodic Table Part A 21102 1990 te - 10090 Calculate the partial pressure of O2 (in mmHg) in a hyperbaric chamber under these conditions. Express your answer using two significant figures. VAp ? Po2 = mmHg I5opsi Submit Request Answer Next Provide Feedback P Pearson Contact Us Terms of Usel Privacy PolicyI Permissions Copyright 2019 Pearson Education Inc. All rights reserved.
Problem 22 22 of 27 > Scuba divers who suffer from decompression sickness are treated in hyperbaric chambers using heliox (21 % Oxygen, 79% helium) at pressures up to 150 psi Review I Constants I Periodic Table Part A 21102 1990 te - 10090 Calculate the partial pressure of O2 (in mmHg) in a hyperbaric chamber under these conditions. Express your answer using two significant figures. VAp ? Po2 = mmHg I5opsi Submit Request Answer Next Provide Feedback P Pearson Contact Us Terms of Usel Privacy PolicyI Permissions Copyright 2019 Pearson Education Inc. All rights reserved.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.111P: 5-111 Diving, particularly SCUBA (Self-Contained Underwater Breathing Apparatus) diving, subjects...
Related questions
Question
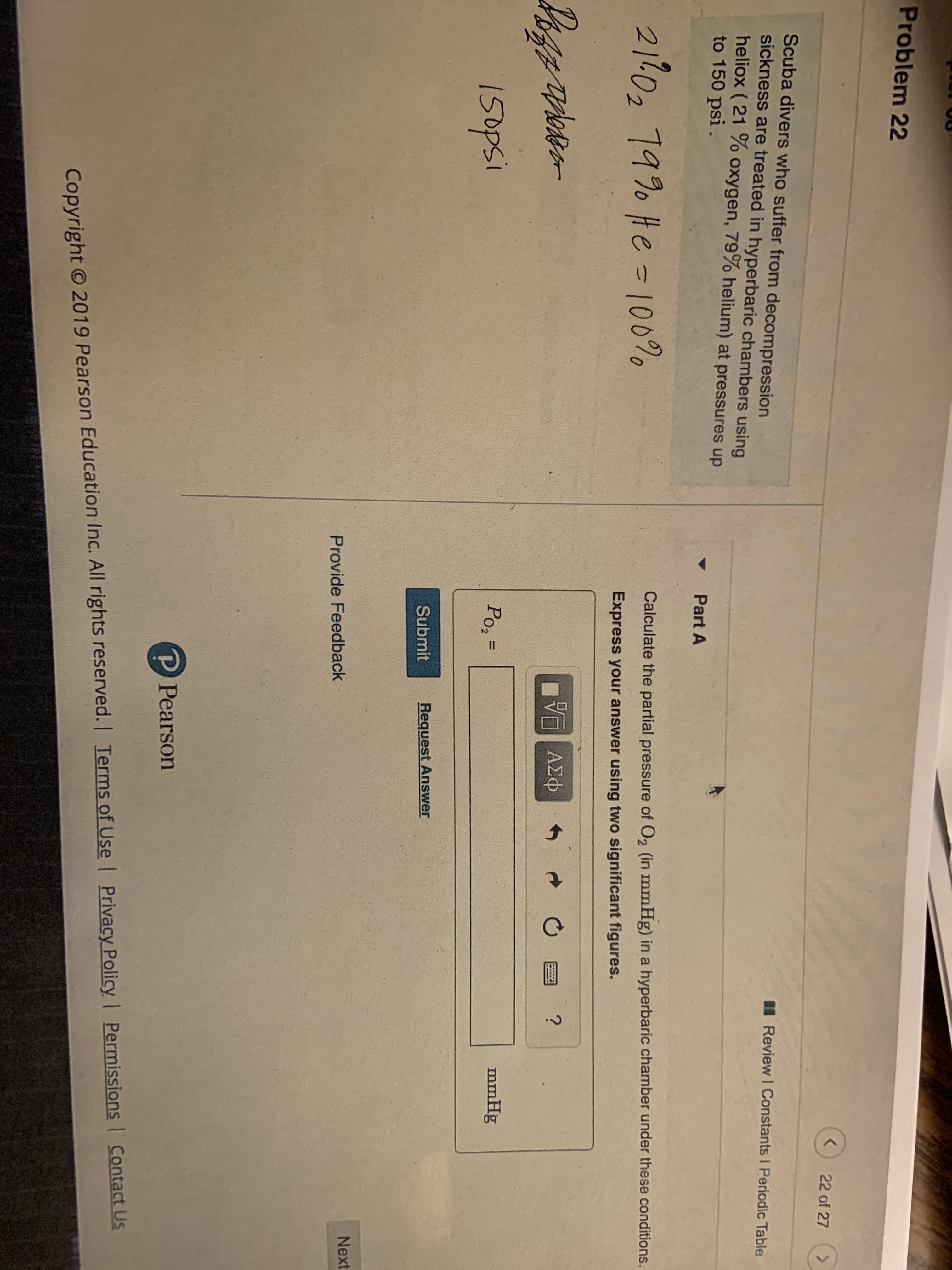
Transcribed Image Text:Problem 22
22 of 27
>
Scuba divers who suffer from decompression
sickness are treated in hyperbaric chambers using
heliox (21 % Oxygen, 79% helium) at pressures up
to 150 psi
Review I Constants I Periodic Table
Part A
21102 1990 te - 10090
Calculate the partial pressure of O2 (in mmHg) in a hyperbaric chamber under these conditions.
Express your answer using two significant figures.
VAp
?
Po2 =
mmHg
I5opsi
Submit
Request Answer
Next
Provide Feedback
P Pearson
Contact Us
Terms of Usel Privacy PolicyI Permissions
Copyright
2019 Pearson Education Inc. All rights reserved.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning