Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 36MP
Related questions
Question
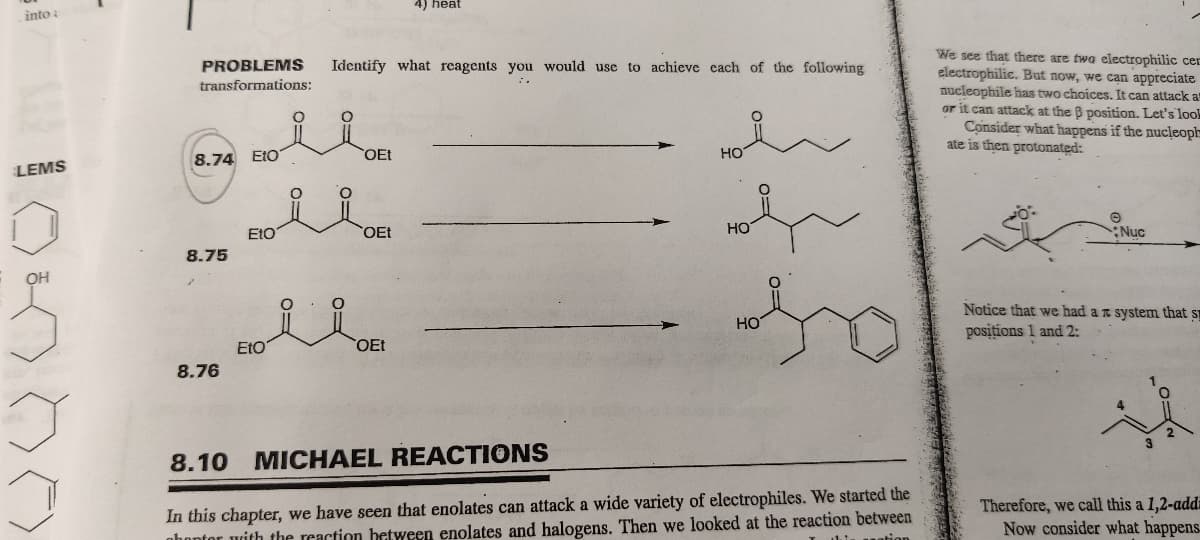
Please do 8.74
Transcribed Image Text:into a
LEMS
OH
J
PROBLEMS Identify what reagents you would use to achieve each of the following
transformations:
8.74 Eto
8.75
8.76
Eto'
Etoi
FO
O
O
OEt
OEt
4) heat
OEt
HO
HO
HO
8.10 MICHAEL REACTIONS
In this chapter, we have seen that enolates can attack a wide variety of electrophiles. We started the
phoptor with the reaction between enolates and halogens. Then we looked at the reaction between
this nation
We see that there are two electrophilic cer
electrophilie. But now, we can appreciate
nucleophile has two choices. It can attack a
or it can attack at the B position. Let's look
Consider what happens if the nucleoph
ate is then protonated:
Nuc
Notice that we had a system that s
positions 1 and 2:
3
2
Therefore, we call this a 1,2-add
Now consider what happens
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning