General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter14: Alcohols, Phenols And Ethers
Section: Chapter Questions
Problem 14.141EP
Related questions
Question
The moleculeshave the same molecular formula 1C3H8O2 but different
chemical structures. (a) Which molecule(s), if any, can
engage in hydrogen bonding? (b) Which molecule do you
expect to have a larger dipole moment? (c) One of these molecules
has a normal boiling point of 97.2 °C, while the other
one has a normal boiling point of 10.8 °C. Assign each molecule
to its normal boiling point. [Sections 11.2 and 11.5]
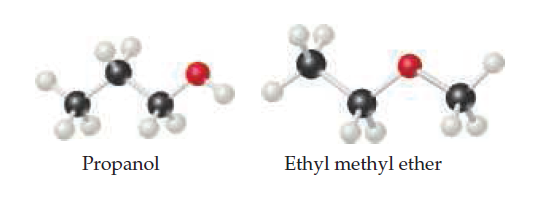
Transcribed Image Text:Propanol
Ethyl methyl ether
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,