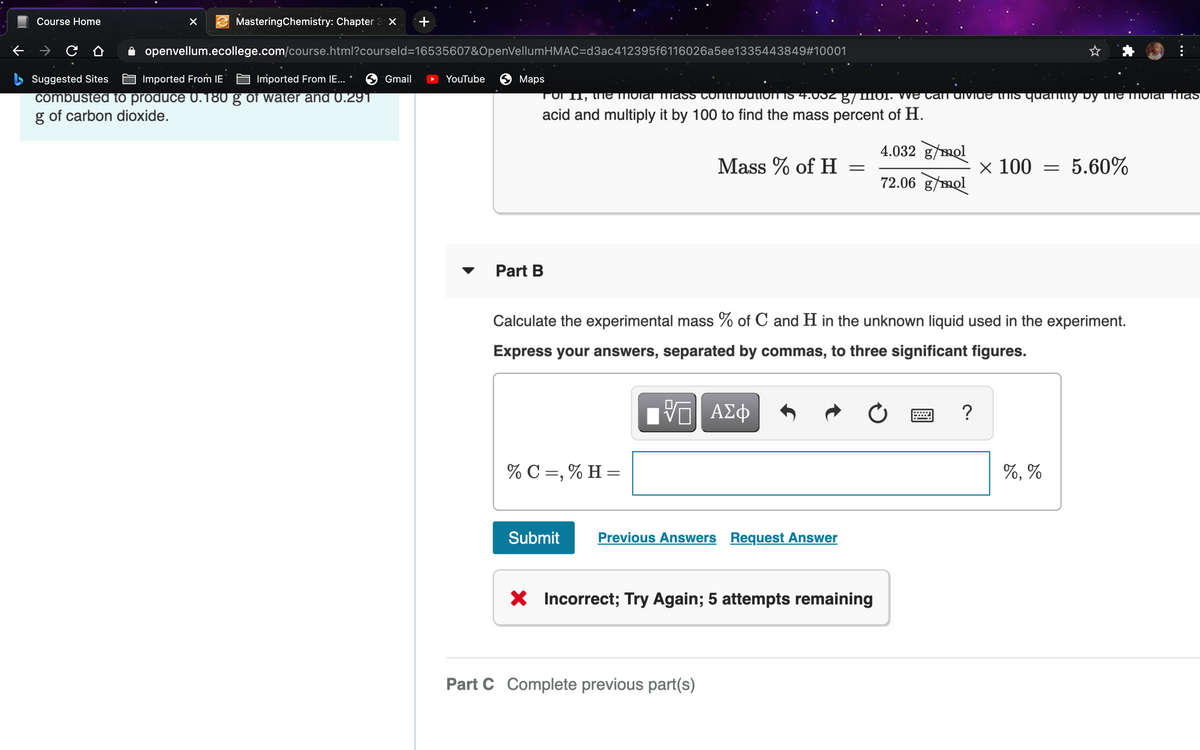
Propenoic acid, C3H4O2C3H4O2, is a reactive organic liquid that is used in the manufacturing of plastics, coatings, and adhesives. An unlabeled container is thought to contain this liquid. A 0.257-gg sample is combusted to produce 0.180 gg of water and 0.291 gg of carbon dioxide.
Propenoic acid, C3H4O2C3H4O2, is a reactive organic liquid that is used in the manufacturing of plastics, coatings, and adhesives. An unlabeled container is thought to contain this liquid. A 0.257-gg sample is combusted to produce 0.180 gg of water and 0.291 gg of carbon dioxide.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Propenoic acid, C3H4O2C3H4O2, is a reactive organic liquid that is used in the manufacturing of plastics, coatings, and adhesives. An unlabeled container is thought to contain this liquid. A 0.257-gg sample is combusted to produce 0.180 gg of water and 0.291 gg of carbon dioxide.
Transcribed Image Text:Course Home
MasteringChemistry: Chapter 3 ×
+
openvellum.ecollege.com/course.html?courseld=16535607&OpenVellumHMAC=d3ac412395f6116026a5ee1335443849#10001
9 Маps
TOI 1, thE TMOlarmaSS COniMbutioNIS 4.USZ g/mo1
O Suggested Sites
O Imported From IE
Imported From IE..
6 Gmail
YouTube
(01. We Can divide tiIS quantity by the mIOlanias
combusted to produce 0.180 g of water and 0.291
g of carbon dioxide.
acid and multiply it by 100 to find the mass percent of H.
4.032 g/mol
Mass % of H
x 100
5.60%
72.06 g/mol
Part B
Calculate the experimental mass % of C and H in the unknown liquid used in the experiment.
Express your answers, separated by commas, to three significant figures.
ΑΣφ
% C =, % H =
%, %
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Part C Complete previous part(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



