Propyl amine can be prepared from the reaction of Ammonia and 1 point Propyl bromide Propanone Propane Propene The typels of amines that exhibit a degree of acidity due to the presence * point of protic Hydrogen islare: (a) primary amine, (b) secondary amine, (c) tertiary amine, (d) quaternary amine a a and b b and c c and d Amines are basic in nature because of the presence of* 1 point its polarity its trigonal pyramidal geometry lone pair in N N-H bond
Propyl amine can be prepared from the reaction of Ammonia and 1 point Propyl bromide Propanone Propane Propene The typels of amines that exhibit a degree of acidity due to the presence * point of protic Hydrogen islare: (a) primary amine, (b) secondary amine, (c) tertiary amine, (d) quaternary amine a a and b b and c c and d Amines are basic in nature because of the presence of* 1 point its polarity its trigonal pyramidal geometry lone pair in N N-H bond
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.31P
Related questions
Question
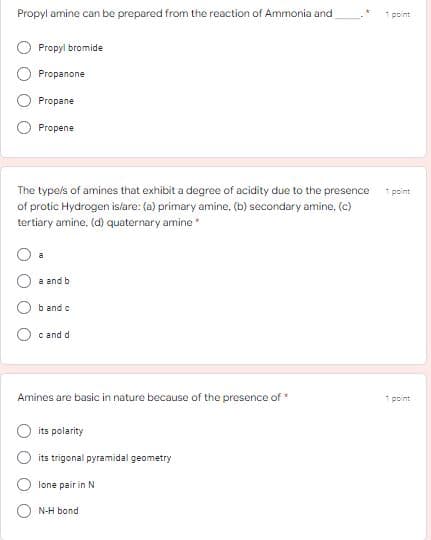
Transcribed Image Text:Propyl amine can be prepared from the reaction of Ammonia and
1 point
Propyi bromide
Propanone
Propane
Propene
The typels of amines that exhibit a degree of acidity due to the presence
* point
of protic Hydrogen islare: (a) primary amine, (b) secondary amine, (c)
tertiary amine, (d) quaternary amine
a
a and b
b and c
c and d
Amines are basic in nature because of the presence of*
1 point
its polarity
its trigonal pyramidal geometry
lone pair in N
N-H bond
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
