Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 10P
Related questions
Question
For question look at picture regarding precision %

Transcribed Image Text:Is the precision of your two trials within 5 %? This means is your % average deviation
<5 %. Support your answer with calculations.
mu tue trigls
was not
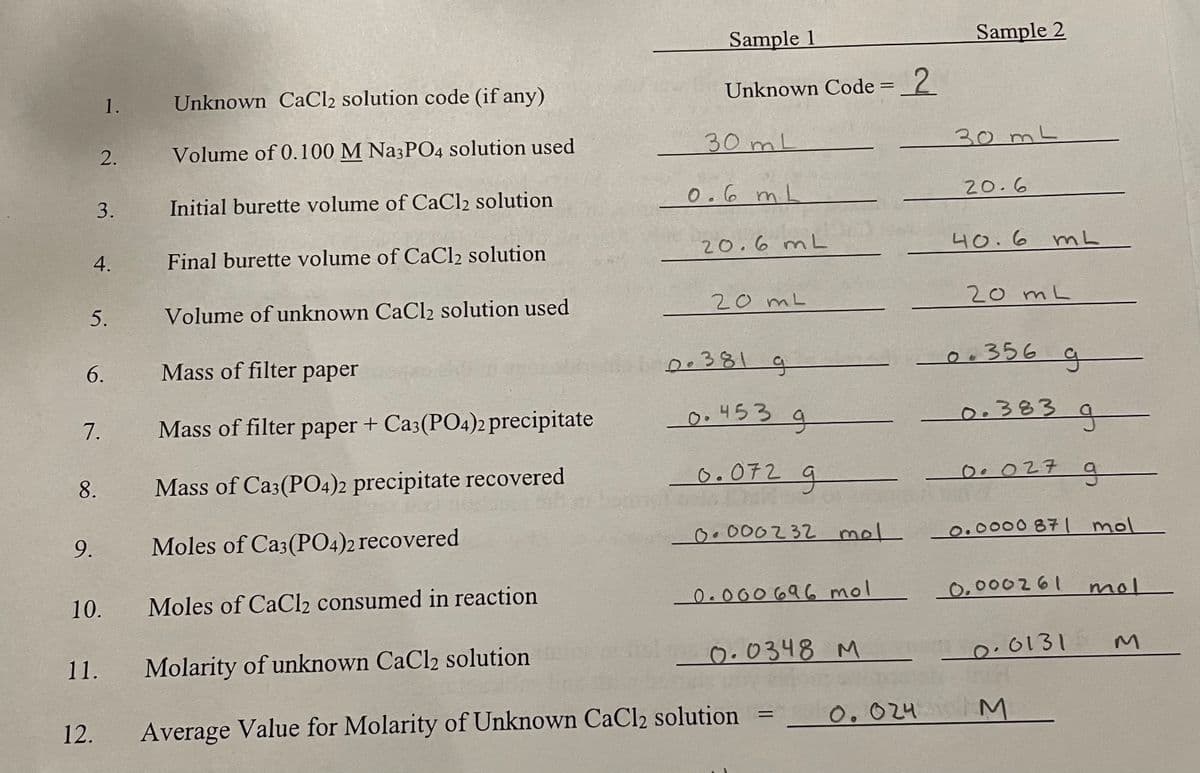
Transcribed Image Text:Sample 1
Sample 2
Unknown CaCl2 solution code (if any)
Unknown Code = _2
%3D
1.
2.
Volume of 0.100 M Na3PO4 solution used
30 mL
30ML
3.
Initial burette volume of CaCl2 solution
0.6 m L
20.6
4.
Final burette volume of CaCl2 solution
20.6 mL
40.6 mL
5.
Volume of unknown CaCl2 solution used
20 mL
20 mL
6.
Mass of filter paper
Do381 g
0:356 q
7.
Mass of filter paper + Ca3(PO4)2 precipitate
0.453 q
0.3839
.३६३
8.
Mass of Ca3(PO4)2 precipitate recovered
O.072
0. 027 a
9.
Moles of Ca3(P04)2 recovered
O. 000232 mel
0.0000 871 mol
10.
Moles of CaCl2 consumed in reaction
0.000 696 mol
0,000261
mol
Molarity of unknown CaCl2 solution
0.0348 M
0.0131
11.
12.
Average Value for Molarity of Unknown CaCl2 solution
0.024
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co