Q: Choose a molecule which displays an exception to the octet rule and illustrate clearly, using Lewis dot structures, why it is considered an exception. Discuss whether resonance structures can account for its stability. A: NO3 is an molecule that does not satisfy the octet rule. The Lewis dot structure for nitrogen trioxide is It does satisfy the octet rule because each atom in the molecule only has access to 7 electrons, instead of the eight required, as shown by the circled atoms. :Ö N.:O:
Q: Choose a molecule which displays an exception to the octet rule and illustrate clearly, using Lewis dot structures, why it is considered an exception. Discuss whether resonance structures can account for its stability. A: NO3 is an molecule that does not satisfy the octet rule. The Lewis dot structure for nitrogen trioxide is It does satisfy the octet rule because each atom in the molecule only has access to 7 electrons, instead of the eight required, as shown by the circled atoms. :Ö N.:O:
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 91E: The most common exceptions to the octet rule are compounds or ions with central atoms having more...
Related questions
Question
Help
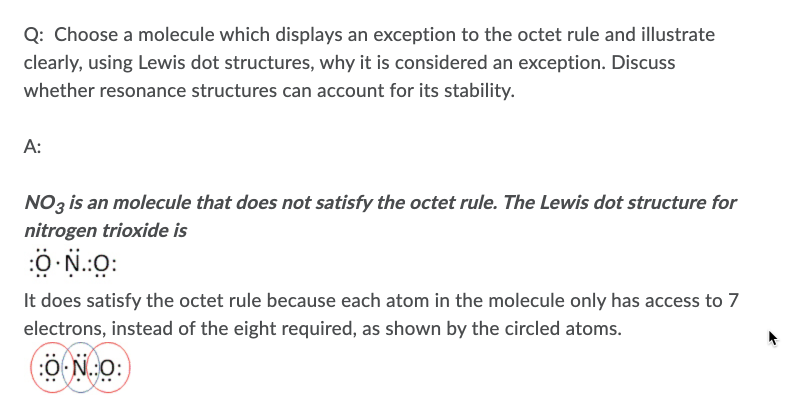
Transcribed Image Text:Q: Choose a molecule which displays an exception to the octet rule and illustrate
clearly, using Lewis dot structures, why it is considered an exception. Discuss
whether resonance structures can account for its stability.
A:
NO3 is an molecule that does not satisfy the octet rule. The Lewis dot structure for
nitrogen trioxide is
:0 N.:O:
It does satisfy the octet rule because each atom in the molecule only has access to 7
electrons, instead of the eight required, as shown by the circled atoms.
(:O N.:O:
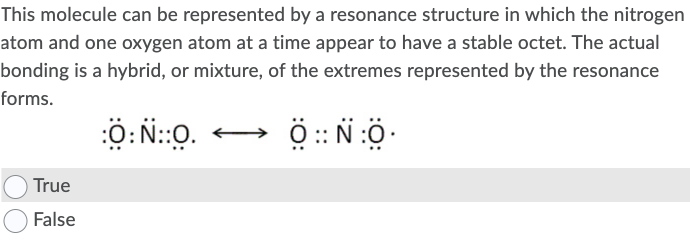
Transcribed Image Text:This molecule can be represented by a resonance structure in which the nitrogen
atom and one oxygen atom at a time appear to have a stable octet. The actual
bonding is a hybrid, or mixture, of the extremes represented by the resonance
forms.
Ö: N::0.
True
False
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning