Types of Chemical Bonds
The attractive force which has the ability of holding various constituent elements like atoms, ions, molecules, etc. together in different chemical species is termed as a chemical bond. Chemical compounds are dependent on the strength of chemical bonds between its constituents. Stronger the chemical bond, more will be the stability in the chemical compounds. Hence, it can be said that bonding defines the stability of chemical compounds.
Polarizability In Organic Chemistry
Polarizability refers to the ability of an atom/molecule to distort the electron cloud of neighboring species towards itself and the process of distortion of electron cloud is known as polarization.
Coordinate Covalent Bonds
A coordinate covalent bond is also known as a dative bond, which is a type of covalent bond. It is formed between two atoms, where the two electrons required to form the bond come from the same atom resulting in a semi-polar bond. The study of coordinate covalent bond or dative bond is important to know about the special type of bonding that leads to different properties. Since covalent compounds are non-polar whereas coordinate bonds results always in polar compounds due to charge separation.
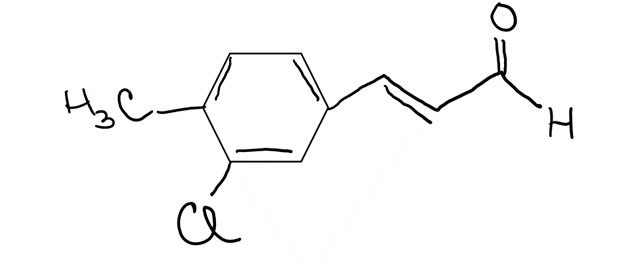
This compound has the formula C6H5CH3ClCH=CHCHO Each intersection of adjoining lines is a carbon atom. =C- or -C-
- a sketch of this molecule showing every hydrogen atom needed to complete octets and any lone pairs of electrons that are needed to complete the oxygen and chlorine atom octets
- Write by the following atoms the VSEPR geometry on the drawing as linear, bent, trigonal planar, bent, trigonal pyramidal or tetrahedral for the atoms:
- The carbon on the right hand side directly bonded to Oxygen
- The carbon that is CH3 on the left side
- The carbons in the ring (all the same)
Step by step
Solved in 2 steps with 1 images


