Q1: calculate the density of solute (10 ml benzene) in liquid mixture if the mole fraction of solvent ( 20 ml carbon tetrachloride and ) 0.342 and the density of solvent (1590 Kg/m³) at 30 °C ? Atwe(C=12,Cl=35.5,H=1) g/ mol
Q1: calculate the density of solute (10 ml benzene) in liquid mixture if the mole fraction of solvent ( 20 ml carbon tetrachloride and ) 0.342 and the density of solvent (1590 Kg/m³) at 30 °C ? Atwe(C=12,Cl=35.5,H=1) g/ mol
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.1: Units Of Concentration
Problem 1CYU: (a) If you dissolve 10.0 g (about one heaping teaspoonful) of sugar (sucrose, C12H22O11) in a cup of...
Related questions
Question
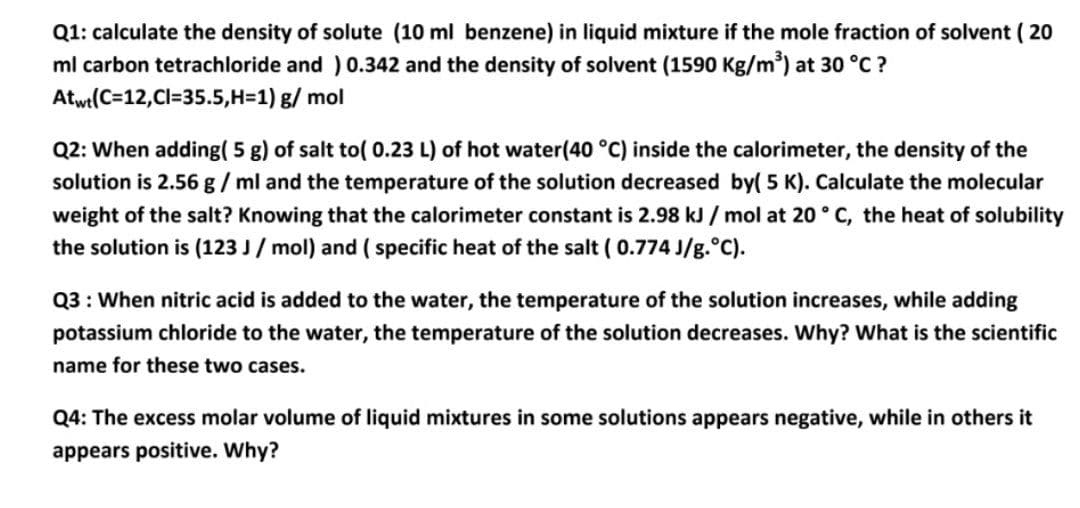
Transcribed Image Text:Q1: calculate the density of solute (10 ml benzene) in liquid mixture if the mole fraction of solvent ( 20
ml carbon tetrachloride and ) 0.342 and the density of solvent (1590 Kg/m) at 30 °C ?
Atwt(C=12,Cl=35.5,H=1) g/ mol
Q2: When adding( 5 g) of salt to( 0.23 L) of hot water(40 °C) inside the calorimeter, the density of the
solution is 2.56 g / ml and the temperature of the solution decreased by( 5 K). Calculate the molecular
weight of the salt? Knowing that the calorimeter constant is 2.98 kJ / mol at 20° C, the heat of solubility
the solution is (123 J / mol) and ( specific heat of the salt ( 0.774 J/g.°C).
Q3 : When nitric acid is added to the water, the temperature of the solution increases, while adding
potassium chloride to the water, the temperature of the solution decreases. Why? What is the scientific
name for these two cases.
Q4: The excess molar volume of liquid mixtures in some solutions appears negative, while in others it
appears positive. Why?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning