What is the solubility of O₂ in water for a Heliox 75/25 gas mixture (75% He and 25% O₂) total pressure of 2.8 am at 25°C.7 M -13x10- 1.36mes10-4rac(Mat for O₂ at 25°C. atm 0.15x10PM 9.1x105M 5.9×10¹ M 19x105M QUESTION 9
What is the solubility of O₂ in water for a Heliox 75/25 gas mixture (75% He and 25% O₂) total pressure of 2.8 am at 25°C.7 M -13x10- 1.36mes10-4rac(Mat for O₂ at 25°C. atm 0.15x10PM 9.1x105M 5.9×10¹ M 19x105M QUESTION 9
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 124CP
Related questions
Question
Question 8 only
Transcribed Image Text:Ogas-liquid
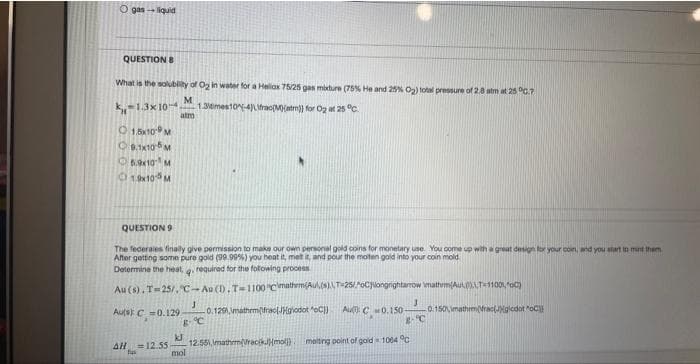
QUESTION B
What is the solubility of O₂ in water for a Hellox 75/25 gas mixture (75% He and 25% O₂) total pressure of 2.0 atm at 25°C.7
M
-1.3x10- 1.36mes10-4)\frac(M)(atm)) for O₂ at 25 °C.
atm
1,5x10 M
9.1x10-5 M
5.9×10 M
19x105 M
QUESTION 9
The federales finally give permission to make our own personal gold coins for monetary use. You come up with a great design for your coin, and you start to mund them
After getting some pure gold (99.99%) you heat it, met it, and pour the moten gold into your coin mold
Determine the heat, q. required for the following process
Au (s). T-25/, C-Au (D.T-1100 "C\mathrm(Aul(s),T-25/AoClongrightarrow \mathem(Aut),T-1100100)
Auist C 2=0.129
0.129,\mathem(frac{godot oC) Au: C0.150- -0.150,\mathrm(rac(gicdot "oC)
J
B-C..
kJ
mol
3
gºC
AH = 12.55 12.551,\mathrm(racik(mol) melting point of goid=1064 °C
fus
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning