Q2. SO parts A reversed reversible heat engine extracts heat 01-22 from a cold reservoir at temperature Told = 270K to supply heat to a rigid pressure vessel filled with a system of wet steam of mass m=10kg. The arrangement is depicted in Fig. Q2, where heat supplied by the reversed engine causes the pressure of the system to rise from p₁ = 2 bar at state point 1 to p₂ = 10 bar at state point 2. Subsequently, the reversed engine is reversed, and heat is extracted from the steam to produce work W2-1, which returns the system back to state point 1, i.e., the system undergoes a cycle. Information recorded at the state points depicted in the figure is as follows: State point 1: dryness fraction x₁ = 0.1 and pressure p₁ = 2 bar. State point 2: p₂ = 10 bar. Assume that the only transfers of heat to take place are those labelled in Fig. Q2, i.e., 01-2, Qoold, Phot and Additionally, assume that the role of the rigid pressure vessel is solely limited to containment, so that any changes to its temperature can be reasonably neglected. The shaft work supplied to the reversed engine during process 1 to 2 is W1-2 = 3000 kJ. (a) Determine the specific internal energy u, and specific volume v₁ of the system of steam at state point 1. (b) Determine the specific internal energy u₂ and dryness fraction ✗2 of the system of steam at state point 2. " (c) Determine the coefficient of performance of the reversed heat engine and hence determine the temperature of a hot reservoir that could reasonably replace the system of steam. (d) Determine the thermal efficiency of the heat engine. H₂O P₁ = 2bar x₁ = 0.1 Reversed heat engine H₂O Rigid pressure vessel containing H2O P2 = 10bar 02-1 W1-2 W2-1 Heat Engine Tcold=270K (a) At state point one heat supply is started. Tcold = 270K (b) At state point two heat extraction is started. Figure Q2. Reversible heat engine used for heating and work (i.e., Qhot and W2-1).
Q2. SO parts A reversed reversible heat engine extracts heat 01-22 from a cold reservoir at temperature Told = 270K to supply heat to a rigid pressure vessel filled with a system of wet steam of mass m=10kg. The arrangement is depicted in Fig. Q2, where heat supplied by the reversed engine causes the pressure of the system to rise from p₁ = 2 bar at state point 1 to p₂ = 10 bar at state point 2. Subsequently, the reversed engine is reversed, and heat is extracted from the steam to produce work W2-1, which returns the system back to state point 1, i.e., the system undergoes a cycle. Information recorded at the state points depicted in the figure is as follows: State point 1: dryness fraction x₁ = 0.1 and pressure p₁ = 2 bar. State point 2: p₂ = 10 bar. Assume that the only transfers of heat to take place are those labelled in Fig. Q2, i.e., 01-2, Qoold, Phot and Additionally, assume that the role of the rigid pressure vessel is solely limited to containment, so that any changes to its temperature can be reasonably neglected. The shaft work supplied to the reversed engine during process 1 to 2 is W1-2 = 3000 kJ. (a) Determine the specific internal energy u, and specific volume v₁ of the system of steam at state point 1. (b) Determine the specific internal energy u₂ and dryness fraction ✗2 of the system of steam at state point 2. " (c) Determine the coefficient of performance of the reversed heat engine and hence determine the temperature of a hot reservoir that could reasonably replace the system of steam. (d) Determine the thermal efficiency of the heat engine. H₂O P₁ = 2bar x₁ = 0.1 Reversed heat engine H₂O Rigid pressure vessel containing H2O P2 = 10bar 02-1 W1-2 W2-1 Heat Engine Tcold=270K (a) At state point one heat supply is started. Tcold = 270K (b) At state point two heat extraction is started. Figure Q2. Reversible heat engine used for heating and work (i.e., Qhot and W2-1).
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
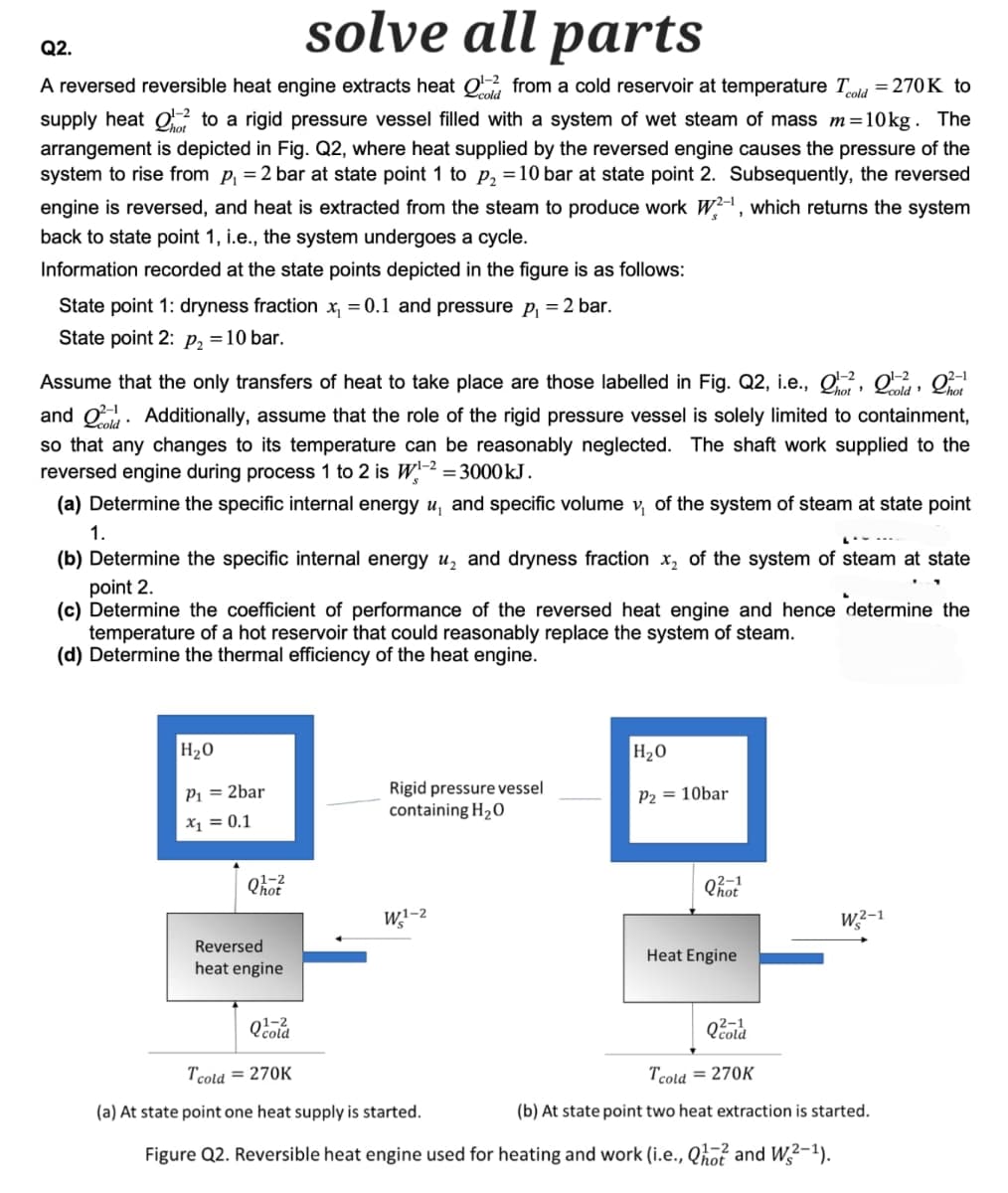
Transcribed Image Text:Q2.
solve all parts
A reversed reversible heat engine extracts heat Q1-22 from a cold reservoir at temperature Tcold = 270K to
supply heat to a rigid pressure vessel filled with a system of wet steam of mass m=10kg. The
arrangement is depicted in Fig. Q2, where heat supplied by the reversed engine causes the pressure of the
system to rise from p₁ = 2 bar at state point 1 to p₂ = 10 bar at state point 2. Subsequently, the reversed
engine is reversed, and heat is extracted from the steam to produce work W2-1, which returns the system
back to state point 1, i.e., the system undergoes a cycle.
Information recorded at the state points depicted in the figure is as follows:
State point 1: dryness fraction ✗₁ = 0.1 and pressure p₁ = 2 bar.
State point 2: P₂ = 10 bar.
2-1
Assume that the only transfers of heat to take place are those labelled in Fig. Q2, i.e., 02, 0,
and Additionally, assume that the role of the rigid pressure vessel is solely limited to containment,
so that any changes to its temperature can be reasonably neglected. The shaft work supplied to the
reversed engine during process 1 to 2 is W1-2 = 3000 kJ.
(a) Determine the specific internal energy u, and specific volume v₁ of the system of steam at state point
1.
(b) Determine the specific internal energy u₂ and dryness fraction ✗2 of the system of steam at state
point 2.
(c) Determine the coefficient of performance of the reversed heat engine and hence determine the
temperature of a hot reservoir that could reasonably replace the system of steam.
(d) Determine the thermal efficiency of the heat engine.
H₂O
P₁ = 2bar
x₁ = 0.1
H₂O
Rigid pressure vessel
containing H₂O
P2 = 10bar
01-2
Reversed
heat engine
Q1-2
Tcold = 270K
W1-2
2-1
Qhot
W2-1
Heat Engine
(a) At state point one heat supply is started.
2-1
Qcold
Tcold = 270K
(b) At state point two heat extraction is started.
Figure Q2. Reversible heat engine used for heating and work (i.e., Q10 and W2-1).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Given
VIEWStep 2: Calculation of specific internal energy and specific volume at state 1
VIEWStep 3: Calculation of specific internal energy and dryness fraction at state 2
VIEWStep 4: Calculation of COP of reversed heat engine and temperature of hot reservoir replace by the system
VIEWSolution
VIEWStep by step
Solved in 5 steps with 22 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY