Q4. There are 4 variables that describe the behavior of a gas. These variables are P (presure), V (volume), T (temperature), and n (number of moles). Which two variables are held constant in Boyle's experiment
Q4. There are 4 variables that describe the behavior of a gas. These variables are P (presure), V (volume), T (temperature), and n (number of moles). Which two variables are held constant in Boyle's experiment
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 24PE: Suppose a gasfilled incandescent light bulb is manufactured so that the gas inside the bulb is at...
Related questions
Question
please solve question 4
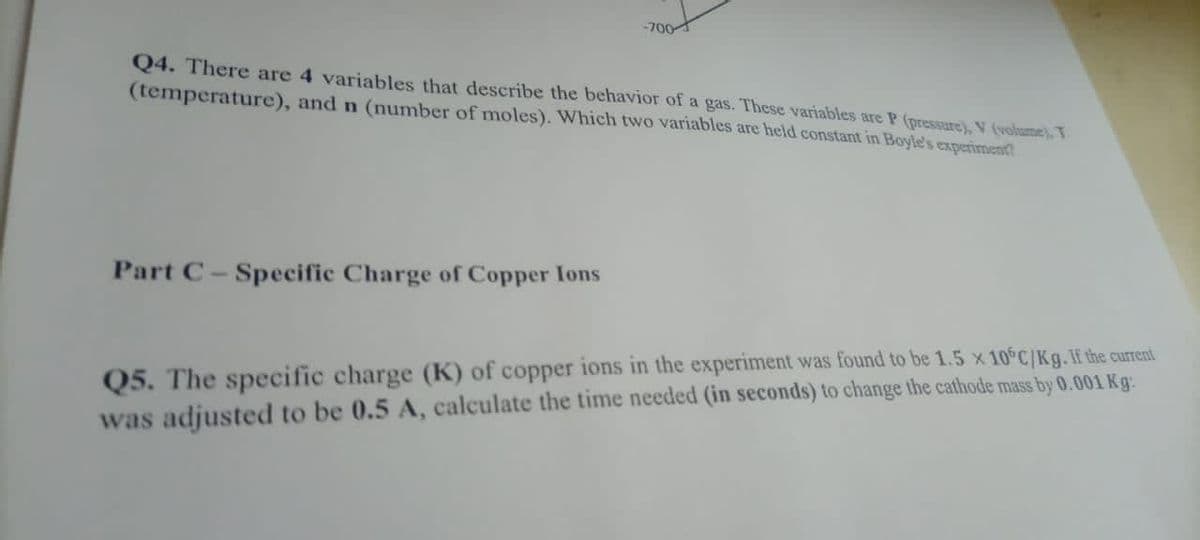
Transcribed Image Text:-700
Q4. There are 4 variables that describe the behavior of a gas. These variables are P (pressure), V (volume). T
(temperature), and n (number of moles). Which two variables are held constant in Boyle's experiment
Part C-Specific Charge of Copper Ions
Q5. The specific charge (K) of copper ions in the experiment was found to be 1.5 x 10 C/Kg.If the current
was adjusted to be 0.5 A, calculate the time needed (in seconds) to change the cathode mass by 0.001 Kg:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning