QIC SA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P10.58). (a) If n moles of an ideal gas are in the cylinder at a temperature of T, use Newton's second law for equilibrium to show that the height h at which the pis- ton is in equilibrium under its own weight is given by Gas nRT mg + PA Figure P10.58 where P, is atmospheric pressure. (b) Is the pressure inside the cylinder less than, equal to, or greater than atmospheric pressure? (c) If the gas in the cylin- der is warmed, how would the answer for h be affected?
QIC SA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P10.58). (a) If n moles of an ideal gas are in the cylinder at a temperature of T, use Newton's second law for equilibrium to show that the height h at which the pis- ton is in equilibrium under its own weight is given by Gas nRT mg + PA Figure P10.58 where P, is atmospheric pressure. (b) Is the pressure inside the cylinder less than, equal to, or greater than atmospheric pressure? (c) If the gas in the cylin- der is warmed, how would the answer for h be affected?
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 81AP: One process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of...
Related questions
Question
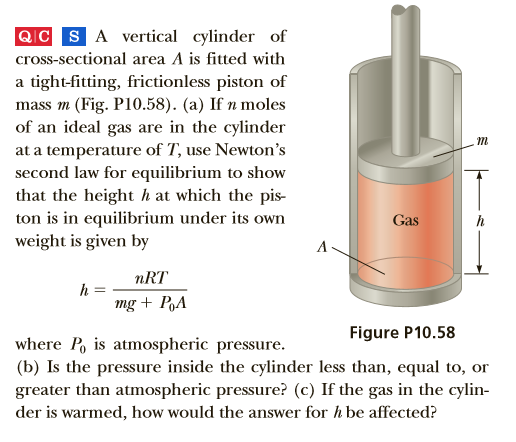
Transcribed Image Text:QIC SA vertical cylinder of
cross-sectional area A is fitted with
a tight-fitting, frictionless piston of
mass m (Fig. P10.58). (a) If n moles
of an ideal gas are in the cylinder
at a temperature of T, use Newton's
second law for equilibrium to show
that the height h at which the pis-
ton is in equilibrium under its own
weight is given by
Gas
nRT
mg + PA
Figure P10.58
where P, is atmospheric pressure.
(b) Is the pressure inside the cylinder less than, equal to, or
greater than atmospheric pressure? (c) If the gas in the cylin-
der is warmed, how would the answer for h be affected?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 6 images

Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University