QMacmillan Learning In scenario C, visible light is in the middle of the yellow region of the visible spectrum. Estimate its wavelength, frequency, and energy per photon. frequency: In scenario D, visible light has a photon energy of 4.347 x 10-19 J. Determine its wavelength, frequency, and color. frequency: s-1 S-1 wavelength: energy per photon: wavelength: The visible light in scenario D is nm
QMacmillan Learning In scenario C, visible light is in the middle of the yellow region of the visible spectrum. Estimate its wavelength, frequency, and energy per photon. frequency: In scenario D, visible light has a photon energy of 4.347 x 10-19 J. Determine its wavelength, frequency, and color. frequency: s-1 S-1 wavelength: energy per photon: wavelength: The visible light in scenario D is nm
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.24QP: Investigating Energy Levels Consider the hypothetical atom X that has one electron like the H atom...
Related questions
Question

Transcribed Image Text:QMacmillan Learning
In scenario C, visible light is in the middle of the yellow
region of the visible spectrum. Estimate its wavelength,
frequency, and energy per photon.
frequency:
In scenario D, visible light has a photon energy of
4.347 x 10-19 J. Determine its wavelength, frequency,
and color.
frequency:
8-1
S-1
wavelength:
energy per photon:
wavelength:
The visible light in scenario D is
nm
J
nm
Transcribed Image Text:Question 11 of 17
.pdf
Learning
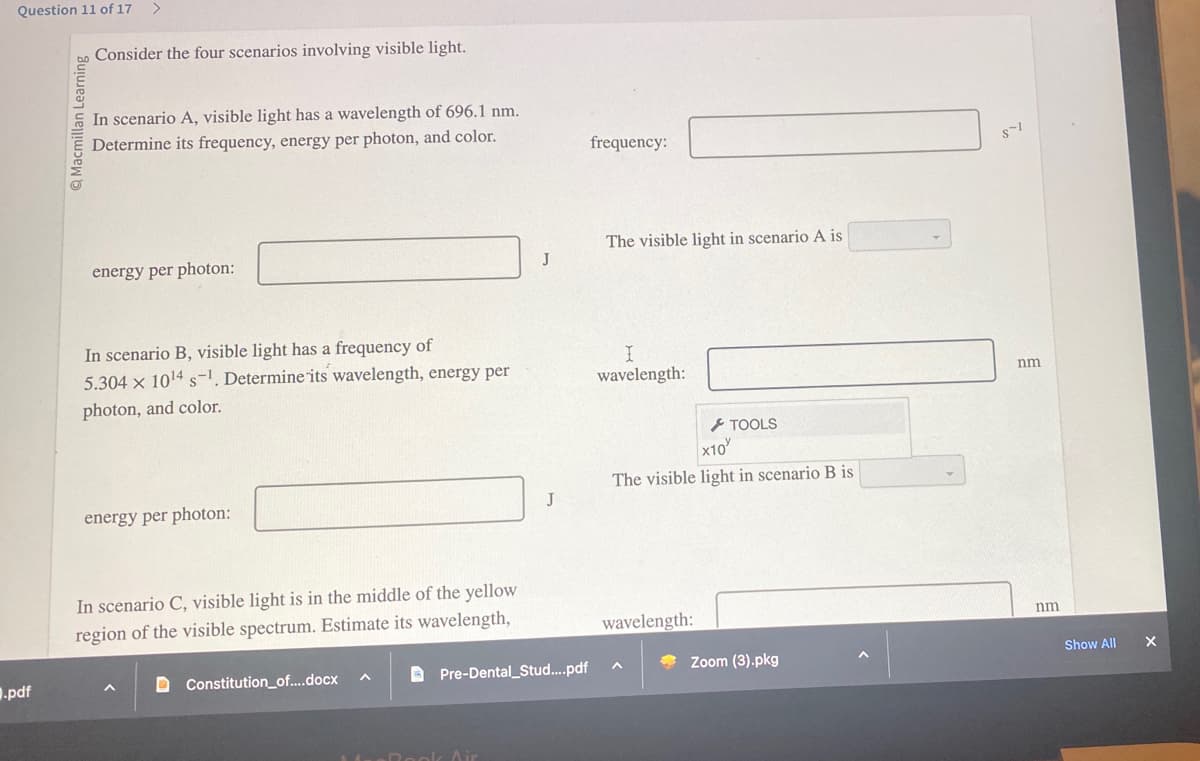
Consider the four scenarios involving visible light.
In scenario A, visible light has a wavelength of 696.1 nm.
Determine its frequency, energy per photon, and color.
energy per photon:
In scenario B, visible light has a frequency of
5.304 x 10¹4 s-¹. Determine its wavelength, energy per
photon, and color.
energy per photon:
In scenario C, visible light is in the middle of the yellow
region of the visible spectrum. Estimate its wavelength,
DConstitution_of....docx
J
Air
J
Pre-Dental Stud.....pdf
frequency:
The visible light in scenario A is
I
wavelength:
x10
The visible light in scenario B is
wavelength:
TOOLS
^
Zoom (3).pkg
S-1
nm
nm
Show All
X
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax