Question 1: Fill in the place holders with the suitable number: Number 1 2 3 4 5 Conductance. split into ions. Voltmeter. Resistivity. Higher concentration. Number Ω m Molar conductivity at infinite dilution. potential difference. Electrolyte. electric current.
Question 1: Fill in the place holders with the suitable number: Number 1 2 3 4 5 Conductance. split into ions. Voltmeter. Resistivity. Higher concentration. Number Ω m Molar conductivity at infinite dilution. potential difference. Electrolyte. electric current.
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 1P
Related questions
Question
Solve question 1 please
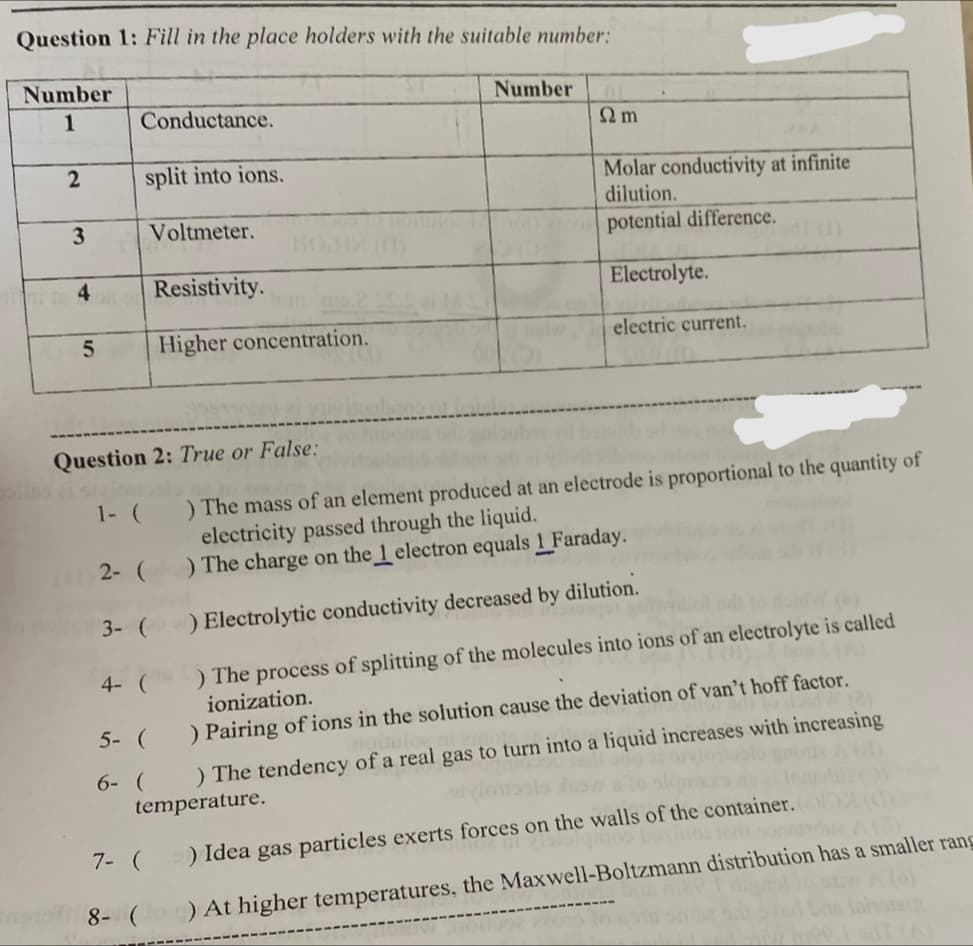
Transcribed Image Text:Question 1: Fill in the place holders with the suitable number:
Number
1
2
3
4
5
Conductance.
split into ions.
(112- (
Voltmeter.
Question 2: True or False:
1- (
3- (
4- (
7-
5- (
6- (
Resistivity.
Higher concentration.
Number
Ω m
Molar conductivity at infinite
dilution.
potential difference.
Electrolyte.
electric current.
) The mass of an element produced at an electrode is proportional to the quantity of
electricity passed through the liquid.
) The charge on the 1 electron equals 1 Faraday.
) Electrolytic conductivity decreased by dilution.
) The process of splitting of the molecules into ions of an electrolyte is called
ionization.
) Pairing of ions in the solution cause the deviation of van't hoff factor.
) The tendency of a real gas to turn into a liquid increases with increasing
20qmosso ili ono zio
temperature.
) Idea gas particles exerts forces on the walls of the container.
8- () At higher temperatures, the Maxwell-Boltzmann distribution has a smaller rang
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

